Carbon nanotube: Difference between revisions
| Line 67: | Line 67: | ||
===Laser ablation=== |
===Laser ablation=== |
||
In the laser ablation process, a pulsed laser vaporizes a graphite target in a high temperature reactor while an inert gas is bled into the chamber |
In the laser ablation process, a pulsed laser vaporizes a graphite target in a high temperature reactor while an inert gas is bled into the chamber. The nanotubes develop on the cooler surfaces of the reactor, as the vaporized carbon condenses. A water-cooled surface may be included in the system to collect the nanotubes. |
||
===Chemical vapor deposition (CVD)=== |
===Chemical vapor deposition (CVD)=== |
||
Revision as of 11:58, 6 October 2006

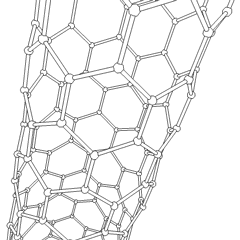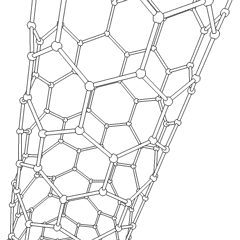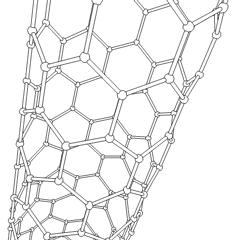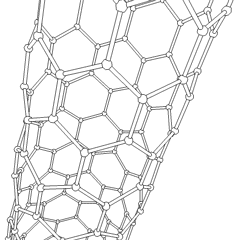
Carbon nanotubes (CNTs) are a recently discovered allotrope of carbon. They take the form of cylindrical carbon molecules and have novel properties that make them potentially useful in a wide variety of applications in nanotechnology, electronics, optics, and other fields of materials science. They exhibit extraordinary strength and unique electrical properties, and are efficient conductors of heat. Inorganic nanotubes have also been synthesized.
A nanotube is a member of the fullerene structural family, which also includes buckyballs. Whereas buckyballs are spherical in shape, a nanotube is cylindrical, with at least one end typically capped with a hemisphere of the buckyball structure. Their name is derived from their size, since the diameter of a nanotube is on the order of a few nanometers (approximately 50,000 times smaller than the width of a human hair), while they can be up to several millimeters in length. There are two main types of nanotubes: single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs).
Manufacturing a nanotube is dependent on applied quantum chemistry, specifically, orbital hybridization. Nanotubes are composed entirely of sp2 bonds, similar to those of graphite. This bonding structure, stronger than the sp3 bonds found in diamond, provides the molecules with their unique strength. Nanotubes naturally align themselves into "ropes" held together by Van der Waals forces. Under high pressure, nanotubes can merge together, trading some sp2 bonds for sp3 bonds, giving great possibility for producing strong, unlimited-length wires through high-pressure nanotube linking.[1]
Discovery
- For a detailed historical outline, see the history section below.
A 2006 editorial written by Marc Monthioux and Vladimir Kuznetsov in the journal Carbon[2] has described the interesting and often misstated origin of the carbon nanotube. A large percentage of academic and popular literature attributes the discovery of hollow, nanometer sized tubes composed of graphitic carbon to Sumio Iijima of NEC in 1991. However, the history of nanometer sized graphitic carbon tubes dates as far back as 1952. In that year, Radushkevich and Lukyanovich published clear images of 50 nanometer diameter tubes made of carbon in the Russian Journal of Physical Chemistry. It is possible that carbon nanotubes were produced before this date, but the invention of the transmission electron microscope allowed the direct visualization of these structures. This discovery was largely unnoticed in the West because of limited information exchange during the Cold War and because the article was published in the Russian language.
Iijima's discovery of carbon nanotubes in the insoluble material of arc-burned graphite rods[3] created the buzz that is now associated with carbon nanotubes. Nanotube research accelerated greatly following the independent discoveries[4][5] by Bethune at IBM[6] and Iijima at NEC of single-wall carbon nanotubes and methods to specifically produce them by adding transition-metal catalysts to the carbon in an arc discharge. The arc discharge technique was well-known to produce the famed Buckminster fullerene on a preparative scale,[7] and these results extended the run of accidental discoveries relating to fullerenes. The original observation of fullerenes in mass spectrometry was not anticipated,[8] and the first mass-production technique by Kratchmer and Huffman was used for several years before realising that it produced fullerenes.[9]
It seemed fitting that nanotubes were also serendipitously discovered. However, a paper by Oberlin, Endo, and Koyama published in 1976 clearly showed hollow carbon fibres with nanometer-scale diameters using a vapour-growth technique.[10] Also, in 1987, Howard G. Tennent of Hyperion Catalysis was issued a U.S. patent for the production of "cylindrical discrete carbon fibrils" with a "constant diameter between about 3.5 and about 70 nanometers…, length 102 times the diameter, and an outer region of multiple essentially continuous layers of ordered carbon atoms and a distinct inner core…."[11] More recently, Endo has been credited with discovering CNTs, and Iijima has been credited for elucidating the structure of NTs. One aspect of the structure of carbon nanotubes is their one-dimensional structure and hollow interior. The one dimensional structure of nanotubes is of great interest to physicists because it permits experiments in one-dimensional quantum physics. The hollow core of carbon nanotubes is also of interest to chemists because it permits encapsulation of molecules, reactions in confined spaces, and controlled release of molecules for applications such as drug delivery.
Types
Single-walled
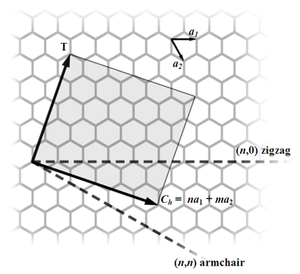
Most SWNTs have a diameter of close to 1 nm, with a tube length that can be many thousands of times larger. SWNTs with length up to orders of centimeters have been produced (Zhu, et al., 2002). The structure of a SWNT can be conceptualized by wrapping a one-atom-thick layer of graphite called graphene into a seamless cylinder. The way the graphene sheet is wrapped is represented by a pair of indices (n,m) called the chiral vector. The integers n and m denote the number of unit vectors along two directions in the honeycomb crystal lattice of graphene. If m=0, the nanotubes are called "zigzag". If n=m, the nanotubes are called "armchair". Otherwise, they are called "chiral".
SWNTs are a very important variety of carbon nanotube because they exhibit important electric properties that are not shared by the multi-walled carbon nanotube (MWNT) variants. SWNTs are the most likely candidate for miniaturizing electronics past the microelectromechanical scale that is currently the basis of modern electronics. The most basic building block of these systems is the electric wire, and SWNTs can be excellent conductors (Dekker, et al., 1999). One useful application of SWNTs is in the development of the first intramolecular field effect transistors (FETs). The production of the first intramolecular logic gate using SWNT FETs has recently become possible as well (Derycke, et al., 2001). To create a logic gate you must have both a p-FET and an n-FET. Because SWNTs are p-FETs when exposed to air and n-FETs when unexposed to oxygen, they were able to protect half of a SWNT from oxygen exposure, while exposing the other half to oxygen. The result was a single SWNT that acted as a NOT logic gate with both p and n-type FETs within the same molecule.
SWNTs are still very expensive to produce, and the development of more affordable synthesis techniques is vital to the future of carbon nanotechnology. If cheaper means of synthesis cannot be discovered, it would make it financially impossible to apply this technology to commercial-scale applications.[citation needed]
Multi-walled
Multiwalled nanotubes (MWNT) consist of multiple layers of graphite rolled in on themselves to form a tube shape. There are two models which can be used to describe the structures of multiwalled nanotubes. In the Russian Doll model, sheets of graphite are arranged in concentric cylinders, eg a (0,8) SWNT within a larger (0,10) SWNT. In the Parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled up newspaper.
Fullerite
Fullerite is a highly incompressible nanotube form. Polymerized single walled nanotubes (P-SWNT) are a class of fullerites and are comparable to diamond in terms of hardness (see also ultrahard fullerite).
Torus
A nanotorus is a carbon nanotube bent into a torus (donut shape). Nanotori have many unique properties, such as magnetic moments 1000 times larger than previously expected for certain specific radii. Many properties such as magnetic moment, thermal stability, etc. vary widely depending on radius of the torus and radius of the tube.
Properties
Strength
Carbon nanotubes are one of the strongest materials known to humans, both in terms of tensile strength and elastic modulus. This strength results from the covalent sp2 bonds formed between the individual carbon atoms. In 2000, an MWNT was tested to have a tensile strength of 63 GPa.[12] In comparison, high-carbon steel has a tensile strength of approximately 1.2 GPa. CNTs also have very high elastic modulus, on the order of 1 TPa.[13] Since carbon nanotubes have a low density for a solid of 1.3-1.4 g/cm³, its specific strength is the best of known materials.
Under excessive tensile strain, the tubes will undergo plastic deformation, which means the deformation is permanent. This deformation begins at strains of approximately 5% [Qian et al, 2002] and can increase the maximum strain the tube undergoes before fracture by releasing strain energy.
CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional or bending stress.
Kinetic
Multiwalled carbon nanotubes, multiple concentric nanotubes precisely nested within one another, exhibit a striking telescoping property whereby an inner nanotube core may slide, almost without friction, within its outer nanotube shell thus creating an atomically perfect linear or rotational bearing.[14][15] This is one of the first true examples of molecular nanotechnology, the precise positioning of atoms to create useful machines. Already this property has been utilized to create the world's smallest rotational motor[16] and a nanorheostat.[17] Future applications such as a gigahertz mechanical oscillator are envisioned.[18]
Electrical
Because of the symmetry and unique electronic structure of graphene, the structure of a nanotube strongly affects its electrical properties. For a given (n,m) nanotube, if 2n + m=3q (where q is an integer), then the nanotube is metallic, otherwise the nanotube is a semiconductor. Thus all armchair (n=m) nanotubes are metallic, and nanotubes (5,0), (6,4), (9,1), etc. are semiconducting. In theory, metallic nanotubes can have an electrical current density more than 1,000 times greater than metals such as silver and copper.
- See also: Fermi point
Thermal
All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as "ballistic conduction," but good insulators laterally to the tube axis.
Defects
As with any material, the existence of defects affects the material properties. Defects can occur in the form of atomic vacancies. High levels of such defects can lower the tensile strength by up to 85%.[19] Another well-known form of defect that occurs in carbon nanotubes is known as the Stone Wales defect, which creates a pentagon and heptagon pair by rearrangement of the bonds. Because of the almost one-dimensional structure of CNTs, the tensile strength of the tube is dependent on the weakest segment of it in a similar manner to a chain, where a defect in a single link diminishes the strength of the entire chain.
The tube's electrical properties are also affected by the presence of defects. A common result is the lowered conductivity through the defective region of the tube. Some defect formation in armchair-type tubes (which are metallic) can cause the region surrounding that defect to become semiconducting. Furthermore single monoatomic vacancies induce magnetic properties.
The tube's thermal properties are heavily affected by defects. Such defects lead to phonon scattering, which in turn increases the relaxation rate of the phonons. This reduces the mean free path, and reduces the thermal conductivity of nanotube structures.
Synthesis
Techniques have been developed to produce nanotubes in sizeable quantities, including arc discharge, laser ablation, high pressure carbon monoxide (HiPco), and chemical vapor deposition (CVD). Most of these processes take place in vacuum or with process gases. CVD growth of CNTs can take place in vacuum or at atmospheric pressure. Large quantities of nanotubes can be synthesized by these methods; advances in catalysis and continuous growth processes are making CNTs more commercially viable.
Arc discharge
Nanotubes were observed in 1991 in the carbon soot of graphite electrodes during an arc discharge that was intended to produce fullerenes. During this process, the carbon contained in the negative electrode sublimates because of the high temperatures caused by the discharge. Because nanotubes were initially discovered using this technique, it has been perhaps the most widely used method of nanotube synthesis.
Laser ablation
In the laser ablation process, a pulsed laser vaporizes a graphite target in a high temperature reactor while an inert gas is bled into the chamber. The nanotubes develop on the cooler surfaces of the reactor, as the vaporized carbon condenses. A water-cooled surface may be included in the system to collect the nanotubes.
Chemical vapor deposition (CVD)

The catalytic vapor phase deposition of carbon was first reported in 1959,[20] but it was not until 1993[21] that carbon nanotubes could be formed by this process.
During CVD, a substrate is prepared with a layer of metal catalyst particles, most commonly nickel, cobalt, iron, or a combination. The diameters of the nanotubes that are to be grown are related to the size of the metal particles. This can be controlled by patterned (or masked) deposition of the metal, annealing, or by plasma etching of a metal layer. The substrate is heated to approximately 700°C. To initiate the growth of nanotubes, two gases are bled into the reactor: a process gas (such as ammonia, nitrogen, hydrogen, etc.) and a carbon-containing gas (such as acetylene, ethylene, ethanol, etc.). Nanotubes grow at the sites of the metal catalyst; the carbon-containing gas is broken apart at the surface of the catalyst particle, and the carbon is transported to the edges of the particle, where it forms the nanotubes. The catalyst particles generally stay at the tips of the growing nanotube during the growth process, although in some cases they remain at the nanotube base, depending on the adhesion between the catalyst particle and the substrate.
If a plasma is generated by the application of a strong electric field during the growth process (plasma enhanced chemical vapor deposition), then the nanotube growth will follow the direction of the electric field.[22] By properly adjusting the geometry of the reactor it is possible to synthesize vertically aligned carbon nanotubes (i.e., perpendicular to the substrate), a morphology that has been of interest to researchers interested in the electron emission from nanotubes. Without the plasma, the resulting nanotubes are often randomly oriented, resembling a bowl of spaghetti. Under certain reaction conditions, even in the absence of a plasma, closely spaced nanotubes will maintain a vertical growth direction resulting in a dense array of tubes resembling a carpet or forest.
Of the various means for nanotube synthesis, CVD shows the most promise for industrial scale deposition in terms of its price/unit ratio. There are additional advantages to the CVD synthesis of nanotubes. Unlike the above methods, CVD is capable of growing nanotubes directly on a desired substrate, whereas the nanotubes must be collected in the other growth techniques. The growth sites are controllable by careful deposition of the catalyst. Additionally, no other growth methods have been developed to produce vertically aligned nanotubes.
Natural, incidental, and controlled flame environments
Fullerenes and carbon nanotubes are not necessarily products of high-tech laboratories; they are commonly formed in such mundane places as ordinary flames,[23] produced by burning methane,[24] ethylene,[25] and benzene,[26] and they have been found in soot from both indoor and outdoor air.[27] However, these naturally occurring varieties can be highly irregular in size and quality because the environment in which they are produced is often highly uncontrolled. Thus, although they can be used in some applications, they can lack in the high degree of uniformity necessary to meet many needs of both research and industry. Recent efforts have focused on producing more uniform carbon nanotubes in controlled flame environments.[28][29][30][31]
Applications
The strength and flexibility of carbon nanotubes makes them of potential use in controlling other nanoscale structures, which suggests they will have an important role in nanotechnology engineering. The highest tensile strength an individual MWNT has been tested to be is 63 GPa.[32] Bulk nanotube materials may never achieve a tensile strength similar to that of individual tubes, but such composites may nevertheless yield strengths sufficient for many applications. Carbon nanotubes have already been used as composite fibers in polymers and concrete to improve the mechanical, thermal and electrical properties of the bulk product.
Structural
- clothes: waterproof tear-resistant cloth fibers
- combat jackets: MIT is working on combat jackets that use carbon nanotubes as ultrastrong fibers and to monitor the condition of the wearer. [3]
- concrete: In concrete, they increase the tensile strength, and halt crack propagation.
- polyethylene: Researchers have found that adding them to polyethylene increases the polymer's elastic modulus by 30%.
- sports equipment: Stronger and lighter tennis rackets, bike parts, golf balls, golf clubs, golf shaft and baseball bats.
- space elevator: If tensile strengths of more than about 70 GPa can be achieved. Monoatomic oxygen in the Earth's upper atmosphere would erode carbon nanotubes at some altitudes, so a space elevator constructed of nanotubes would need to be protected (by some kind of coating). Carbon nanotubes in other applications would generally not need such surface protection.
- ultrahigh-speed flywheels: The high strength/weight ratio enables very high speeds to be achieved.
Electromagnetic
- artificial muscles[33]
- buckypaper - a thin sheet made from nanotubes that are 250 times stronger than steel and 10 times lighter that could be used as a heat sink for chipboards, a backlight for LCD screens or as a faraday cage to protect electrical devices/aeroplanes.
- chemical nanowires: Carbon nanotubes additionally can also be used to produce nanowires of other chemicals, such as gold or zinc oxide. These nanowires in turn can be used to cast nanotubes of other chemicals, such as gallium nitride. These can have very different properties from CNTs - for example, gallium nitride nanotubes are hydrophilic, while CNTs are hydrophobic, giving them possible uses in organic chemistry that CNTs could not be used for.
- computer circuits: A nanotube formed by joining nanotubes of two different diameters end to end can act as a diode, suggesting the possibility of constructing electronic computer circuits entirely out of nanotubes. Because of their good thermal properties, CNTs can also be used to dissipate heat from tiny computer chips. The longest electricity conducting circuit is a fraction of an inch long.(Source: June 2006 National Geographic).
- conductive films: A 2005 paper in Science notes that drawing transparent high strength swathes of SWNT is a functional production technique (Zhang et. al., vol. 309, p. 1215). Additionally, Eikos Inc.[34] of Franklin, Massachusetts is developing transparent, electrically conductive films of carbon nanotubes to replace indium tin oxide (ITO) in LCDs, touch screens, and photovoltaic devices. Carbon nanotube films are substantially more mechanically robust than ITO films, making them ideal for high reliability touch screens and flexible displays. Nanotube films show promise for use in displays for computers, cell phones, PDAs, and ATMs.
- electric motor brushes: Conductive carbon nanotubes have been used for several years in brushes for commercial electric motors. They replace traditional carbon black, which is mostly impure spherical carbon fullerenes. The nanotubes improve electrical and thermal conductivity because they stretch through the plastic matrix of the brush. This permits the carbon filler to be reduced from 30% down to 3.6%, so that more matrix is present in the brush. Nanotube composite motor brushes are better-lubricated (from the matrix), cooler-running (both from better lubrication and superior thermal conductivity), less brittle (more matrix, and fiber reinforcement), stronger and more accurately moldable (more matrix). Since brushes are a critical failure point in electric motors, and also don't need much material, they became economical before almost any other application.
- light bulb filament: alternative to tungsten filaments in incandescent lamps.
- magnets: MWNTs coated with magnetite
- optical ignition: A layer of 29% iron enriched SWNT is placed on top of a layer of explosive material such as PETN, and can be ignited with a regular camera flash.
- solar cells: GE's carbon nanotube diode has a photovoltaic effect. Nanotubes can replace ITO in some solar cells to act as a transparent conductive film in solar cells to allow light to pass to the active layers and generate photocurrent.
- superconductor: Nanotubes have been shown to be superconducting at low temperatures.
- ultracapacitors: MIT is researching the use of nanotubes bound to the charge plates of capacitors in order to dramatically increase the surface area and therefore energy storage ability.[35]
- displays: One use for nanotubes that has already been developed is as extremely fine electron guns, which could be used as miniature cathode ray tubes in thin high-brightness low-energy low-weight displays. This type of display would consist of a group of many tiny CRTs, each providing the electrons to hit the phosphor of one pixel, instead of having one giant CRT whose electrons are aimed using electric and magnetic fields. These displays are known as field emission displays (FEDs).
- transistor: developed at Delft, IBM, and NEC.
Chemical
- air pollution filter: Future applications of nanotube membranes include filtering carbon dioxide from power plant emissions.[36]
- biotech container: Nanotubes can be opened and filled with materials such as biological molecules, raising the possibility of applications in biotechnology.
- water filter: Recently nanotube membranes have been developed for use in filtration. This technique can purportedly reduce desalination costs by 75%. The tubes are so thin that small particles (like water molecules) can pass through them, while larger particles (such as the chloride ions in salt) are blocked.
Mechanical
- oscillator: fastest known oscillators (> 50 GHz).
- liquid flow array: Liquid flows up to five orders of magnitude faster than predicted through array.
- slick surface: slicker than Teflon and waterproof.
In electrical circuits
Carbon nanotubes have many properties—from their unique dimensions to an unusual current conduction mechanism—that make them ideal components of electrical circuits. Currently, there is no reliable way to arrange carbon nanotubes into a circuit.
The major hurdles that must be jumped for carbon nanotubes to find prominent places in circuits relate to fabrication difficulties. The production of electrical circuits with carbon nanotubes are very different from the traditional IC fabrication process. The IC fabrication process is somewhat like sculpture - films are deposited onto a wafer and pattern-etched away. Because carbon nanotubes are fundamentally different from films, carbon nanotube circuits can so far not be mass produced.
Researchers sometimes resort to manipulating nanotubes one-by-one with the tip of an atomic force microscope in a painstaking, time-consuming process. Perhaps the best hope is that carbon nanotubes can be grown through a chemical vapor deposition process from patterned catalyst material on a wafer, which serve as growth sites and allow designers to position one end of the nanotube. During the deposition process, an electric field can be applied to direct the growth of the nanotubes, which tend to grow along the field lines from negative to positive polarity. Another way for the self assembly of the carbon nanotube transistors consist in using chemical or biological techniques to place the nanotubes from solution to determinate place on a substrate.
Even if nanotubes could be precisely positioned, there remains the problem that, to this date, engineers have been unable to control the types of nanotubes—metallic, semiconducting, single-walled, multi-walled—produced. A chemical engineering solution is needed if nanotubes are to become feasible for commercial circuits.
As fiber and film
One application for nanotubes that is currently being researched is high tensile strength fibers. Two methods are currently being tested for the manufacture of such fibers. A French team has developed a liquid spun system that involves pulling a fiber of nanotubes from a bath which yields a product that is approximately 60% nanotubes[citation needed]. The other method, which is simpler but produces weaker fibers uses traditional melt-drawn polymer fiber techniques with nanotubes mixed in the polymer. After drawing, the fibers can have the polymer component burned out of them leaving only the nanotube or they can be left as they are.
Ray Baughman's group from the NanoTech Institute at University of Texas at Dallas produced the current toughest material known in mid-2003 by spinning fibers of single wall carbon nanotubes with polyvinyl alcohol. Beating the previous contender, spider silk, by a factor of four, the fibers require 600 J/g to break[37] In comparison, the bullet-resistant fiber Kevlar is 27–33 J/g. In mid-2005 Baughman and co-workers from Australia's Commonwealth Scientific and Industrial Research Organization developed a method for producing transparent carbon nanotube sheets 1/1000th the thickness of a human hair capable of supporting 50,000 times their own mass. In August 2005, Ray Baughman's team managed to develop a fast method to manufacture up to seven meters per minute of nanotube tape.[38] Once washed with ethanol, the ribbon is only 50 nanometers thick; a square kilometer of the material would only weigh 30 kilograms.
In 2004 Alan Windle's group of scientists at the Cambridge-MIT Institute developed a way to make carbon nanotube fiber continuously at the speed of several centimetres per second just as nanotubes are produced. One thread of carbon nanotubes was more than 100 metres long. The resulting fibers are electrically conductive and as strong as ordinary textile threads.[39][40]
History

- 1952
- Radushkevich and Lukyanovich publish a paper in the Russian Journal of Physical Chemistry showing hollow graphitic carbon fibers that are 50 nanometers in diameter.
- 1976
- Oberlin, Endo and Koyama report CVD growth of nanometer-scale carbon fibers.
- 1979
- Arthur C. Clarke's science fiction novel The Fountains of Paradise popularizes the idea of a space elevator using "a continuous pseudo-one dimensional diamond crystal".[41][42]
- 1985
- 1987
- Howard G. Tennent of Hyperion Catalysis issued a U.S. patent for graphitic, hollow core "fibrils"
- 1991
- Nanotubes discovered in the soot of arc discharge at NEC, by Japanese researcher Sumio Iijima.[43]
- August - Nanotubes discovered in CVD by Al Harrington and Tom Maganas of Maganas Industries, leading to development of a method to synthesize monomolecular thin film nanotube coatings.[44]
- 1993
- Groups led by Donald S. Bethune at IBM and Sumio Iijima at NEC independently discover single-wall carbon nanotubes and methods to produce them using transition-metal catalysts.[45][46]
- 1998
- Nanotube transistor created at Delft and IBM.
- 2001
- April - IBM announces a technique for automatically developing pure semiconductor surfaces from nanotubes.
- 2002
- January - Multi-walled nanotubes demonstrated to be fastest known oscillators (> 50 GHz).[47]
- REBO method of quickly and accurately modeling classical nanotube behavior is described.[48]
- 2003
- April - Demonstration proves that bending changes resistance.[49]
- June - High purity (20% impure) nanotubes with metallic properties were reported to be extracted with electrophoretic techniques.[50]
- September - NEC announced stable fabrication technology of carbon nanotube transistors[51]
- As of 2003, nanotubes cost from 20 euro per gram to 1000 euro per gram, depending on purity, composition (single-wall, double-wall, multi-wall) and other characteristics.
- 2004
- June - Scientists from China's Tsinghua University and Louisiana State University demonstrated the use of nanotubes in incandescent lamps, replacing a tungsten filament in a lightbulb with a carbon nanotube one.
- March - Nature published a photo of an individual 4 cm long single-wall nanotube (SWNT).
- August - Varying the applied voltage emits light at different points along a tube.[52]
- 2005
- May - A prototype high-definition 10-centimetre flat screen made using nanotubes was exhibited[53]
- August - University of California finds Y-shaped nanotubes to be ready-made transistors[54]
- August - General Electric announced the development of an ideal carbon nanotube diode that operates at the "theoretical limit" (the best possible performance). A photovoltaic effect was also observed in the nanotube diode device that could lead to breakthroughs in solar cells, making them more efficient and thus more economically viable.[55]
- August - Nanotube sheet synthesised with dimensions 5 × 100 cm.[56]
- September - Applied Nanotech (Texas), in conjunction with six Japanese electronics firms, have created a prototype of a 25-inch TV using carbon nanotubes. The prototype TV does not suffer from "ghosting," as some types of digital TVs do.
- September - Researchers at Lawrence Livermore National Laboratory demonstrated that ignition by a conventional flashbulb takes place when a layer of 29% iron enriched SWNT is placed on top of a layer of explosive material such as PETN. With ordinary explosives optical ignition is only possible with high powered lasers.[57]
- September - Researchers demonstrated a new way to coat MWNT's with magnetite which after orientation in a magnetic field were able to attract each other over a distance of at least 10 micrometres.[58] The nanotubes were functionalized with negatively charged carboxylic acid groups in an AIBN type free radical addition. Magnetite nanoparticles prepared by the Massart method were given a positive charge by washing with nitric acid which made them stick to the nanotubes by electrostatic forces.
- September - American and Korean scientists, working at Columbia University and Pohang University of Science and Technology and lead by Professor's Philip Kim of Columbia and Kim Kwang-Soo of Pohang, succeeded in pulling out a nested tube from a multiwalled nanotube (MWNT).[59]
- October - Scientists at Florida State University begin investigating applications for buckypaper.
- November - Liquid flows up to five orders of magnitude faster than predicted through array[60]
- December - Indian Institutes of Technology Kanpur(India) announces presence of CNT in Soft-Kohl[61]
- Industry reports indicate nanotube production will increase by 10 to 100 times in the next five years for different types and purity of nanotubes.
- 2006

- January - Thin films of nanotubes made by evaporation[62]
- January - Another new method for growing forests of nanotubes is announced[63]
- January - Elasticity increased from 20% to 280% by raising temperatures, causing diameter and conductivity to change greatly[64][65]
- March - IBM announces that they have built an electronic circuit around a CNT[66][67][68]
- March - Nanotubes used as a scaffold for damaged nerve regeneration[69]
- May - Method of placing nanotube accurately is developed by IBM[70]
- June - Gadget invented by Rice University that can sort nanotubes by size and electrical properties[71]
- July - Nanotubes were alloyed into the carbon fiber bike that won the 2006 Tour de France[72]
- August - ocillating nanotubes found to detect and identify individual molecules[73]
- Prices halve in one year to €1.67 per gram in quantities of 1 kg as MWNT, >50 nm diameter, 50 micrometers long.[74]
References
- ^ http://www.ncnr.nist.gov/staff/taner/nanotube/interlink.pdf
- ^ http://www.cemes.fr/fichpdf/GuestEditorial.pdf
- ^ http://www.nature.com/nature/journal/v354/n6348/abs/354056a0.html
- ^ http://www.nature.com/nature/journal/v363/n6430/abs/363605a0.html
- ^ http://www.nature.com/nature/journal/v363/n6430/abs/363603a0.html
- ^ http://www.almaden.ibm.com/st/nanoscale_science/past/nanotubes/
- ^ http://www.nature.com/nature/journal/v347/n6291/abs/347354a0.html
- ^ http://www.nature.com/nature/journal/v318/n6042/abs/318162a0.html
- ^ http://www.nature.com/nature/journal/v347/n6291/abs/347354a0.html
- ^ A. Oberlin, M. Endo, and T. Koyama, J. Cryst. Growth, 1976, 32, 335.
- ^ http://www.freepatentsonline.com/4663230.html
- ^ http://sciencemag.org/cgi/content/abstract/287/5453/637
- ^ http://ipn2.epfl.ch/CHBU/papers/ourpapers/Forro_NT99.pdf
- ^ http://dx.doi.org/10.1126/science.289.5479.505e
- ^ http://dx.doi.org/10.1126/science.289.5479.602
- ^ http://dx.doi.org/10.1038/nature01823
- ^ http://dx.doi.org/10.1103/PhysRevLett.93.086801
- ^ http://focus.aps.org/story/v9/st4
- ^ http://lib.tkk.fi/Diss/2004/isbn9512273799/article5.pdf
- ^ P. L. Walker Jr. et al., J. Phys. Chem. 63, 133 (1959).
- ^ M. José-Yacamán et al., Appl. Phys. Lett. 62, 657 (1993).
- ^ Z. F. Ren et al., Science 282, 1105 (1998).
- ^ J.M. Singer, J. Grumer, Proc. Combust. Inst. 7, 559 (1959).
- ^ Yuan, Liming (2001). "Nanotubes from methane flames". Chemical physics letters. 340: 237–241. doi:10.1016/S0009-2614(01)00435-3.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Yuan, Liming (2001). "Ethylene flame synthesis of well-aligned multi-walled carbon nanotubes". Chemical physics letters. 346: 23–28. doi:10.1016/S0009-2614(01)00959-9.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Duan, H. M. (1994). "Nanoclusters Produced in Flames". Journal of Physical Chemistry. 98 (49): 12815–12818. doi:10.1021/j100100a001.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Murr, L. E. (2004). "Carbon nanotubes, nanocrystal forms, and complex nanoparticle aggregates in common fuel-gas combustion sources and the ambient air". Journal of Nanoparticle Research. 6: 241–251. doi:10.1023/B:NANO.0000034651.91325.40.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ R.L. Vander Wal, Combust. Flame 130 37-47 (2002).
- ^ A.V. Saveliev, W. Merchan-Merchan, L.A. Kennedy, Combust. Flame 135, 27-33 (2003).
- ^ M.J. Height, J.B. Howard, J.W. Tester, J.B. Vander Sande, Carbon 42, 2295-2307 (2004).
- ^ S. Sen, I.K. Puri, Nanotechnology 15, 264-268 (2004).
- ^ http://sciencemag.org/cgi/content/abstract/287/5453/637
- ^ http://www.newscientisttech.com/channel/tech/dn8859.html
- ^ http://www.eikos.com
- ^ http://lees-web.mit.edu/public/In_the_News/wtr_16326,303,p1.pdf
- ^ http://www.contracostatimes.com/mld/cctimes/news/breaking_news/14612073.htm
- ^ . Super-tough carbon-nanotube fibres Alan B. Dalton et. al. Nature 423, 703 (12 June 2003)|doi:10.1038/423703a
- ^ http://www.worldchanging.com/archives/003330.html
- ^ http://news.bbc.co.uk/2/hi/science/nature/3872931.stm
- ^ http://www.newscientist.com/news/news.jsp?id=ns99994769
- ^ "1D Diamond Crystal", [1]
- ^ Audacious & Outrageous: Space Elevators, [2], NASA, 7 September 2000
- ^ http://www.nature.com/nature/journal/v354/n6348/abs/354056a0.html
- ^ http://patft.uspto.gov/netacgi/nph-Parser?u=%2Fnetahtml%2Fsrchnum.htm&Sect1=PTO1&Sect2=HITOFF&p=1&r=1&l=50&f=G&d=PALL&s1=5143745.PN.&OS=PN/5143745&RS=PN/5143745
- ^ http://www.nature.com/nature/journal/v363/n6430/abs/363605a0.html
- ^ http://www.nature.com/nature/journal/v363/n6430/abs/363603a0.html
- ^ http://focus.aps.org/story/v9/st4
- ^ http://sinnott.mse.ufl.edu/sub05b_nanomechanics.html
- ^ http://focus.aps.org/story/v11/st15
- ^ http://physicsweb.org/article/news/7/6/19/1
- ^ http://www.nec.co.jp/press/en/0309/1901.html
- ^ http://focus.aps.org/story/v14/st8
- ^ http://www.newscientisttech.com/article/mg18625006.800
- ^ http://www.newscientisttech.com/article/dn7847
- ^ http://www.research.ge.com/04_media/news/20050819_cnd.shtml
- ^ http://nanotechweb.org/articles/news/4/8/13/1
- ^ http://pubs3.acs.org/acs/journals/doilookup?in_doi=10.1021/ja0547127
- ^ http://www.rsc.org/Publishing/Journals/CC/article.asp?doi=b506758h
- ^ http://pico.phys.columbia.edu/pdf_papers/PNAS_102_2005_BH.pdf
- ^ http://nanotechweb.org/articles/news/4/11/8/1
- ^ http://www.thenanotechnologygroup.org/index.cfm?Content=88&PressID=691
- ^ http://nanotechweb.org/articles/news/5/1/16/1
- ^ http://nanotechweb.org/articles/news/5/1/4/1
- ^ http://nanotechweb.org/articles/news/5/1/12/1
- ^ http://cnanotech.com/download_files/Issued_Patents/US06986876.pdf
- ^ http://money.cnn.com/2006/03/24/technology/ibm_semiconductor/index.htm
- ^ http://www.newscientisttech.com/article/dn8888
- ^ http://news.bbc.co.uk/1/hi/sci/tech/4839088.stm
- ^ Optic nerve regrown with a nanofibre scaffold
- ^ http://www.newscientisttech.com/article/dn9241
- ^ http://www.newscientisttech.com/article.ns?id=dn9419&feedId=online-news_rss20
- ^ http://news.com.com/Carbon+nanotubes+enter+Tour+de+France/2100-11395_3-6091347.html?tag=fd_carsl
- ^ http://www.newscientisttech.com/article/dn9857-carbonnanotube-strings-may-id-single-molecules.html
- ^ http://www.cheaptubesinc.com/
External links and sources
- New Scientist Special Report - a collection of nanotechnology articles, most on nanotubes
- The stuff of dreams - CNET
- The Nanotube site - Last updated 2006.05.18
- Nanowerk - Information on carbon nanotubes
- Animation of a (29,0) being struck by 10 sets of 9 Argon atoms at 10 eV each (opens in media player)
- The wonderous World of Carbon Nanotubes (In .pdf format, good introduction to nanotube)
- nanotechweb.org nanotube and nanotechnology news and information
- Carbon - Super Stuff Educational interactive with narration and 3D-models of nanotube, diamond, graphite and coal.
- Carbon nanotube on arxiv.org
