Abundance of elements in Earth's crust: Difference between revisions
Appearance
Content deleted Content added
Reverted 2 edits by 106.79.207.8 (talk): Revert test, restore correct formatting (TW) |
correct position of tungsten re: apparent error in Barbalace |
||
| Line 239: | Line 239: | ||
|- |
|- |
||
| 19 |
| 19 |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| 1.25 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| 23 |
| 23 |
||
| [[vanadium]] |
| [[vanadium]] |
||
| Line 260: | Line 249: | ||
|76,000 |
|76,000 |
||
|- |
|- |
||
| |
| 20 |
||
| 17 |
| 17 |
||
| [[chlorine]] |
| [[chlorine]] |
||
| Line 271: | Line 260: | ||
| |
| |
||
|- |
|- |
||
| |
| 21 |
||
| 24 |
| 24 |
||
| [[chromium]] |
| [[chromium]] |
||
| Line 282: | Line 271: | ||
|26,000,000 |
|26,000,000 |
||
|- |
|- |
||
| |
| 22 |
||
| 37 |
| 37 |
||
| [[rubidium]] |
| [[rubidium]] |
||
| Line 293: | Line 282: | ||
| |
| |
||
|- |
|- |
||
| |
| 23 |
||
| 28 |
| 28 |
||
| [[nickel]] |
| [[nickel]] |
||
| Line 304: | Line 293: | ||
|2,250,000 |
|2,250,000 |
||
|- |
|- |
||
| |
| 24 |
||
| 30 |
| 30 |
||
| [[zinc]] |
| [[zinc]] |
||
| Line 315: | Line 304: | ||
|11,900,000 |
|11,900,000 |
||
|- |
|- |
||
| |
| 25 |
||
| 29 |
| 29 |
||
| [[copper]] |
| [[copper]] |
||
| Line 326: | Line 315: | ||
|19,400,000 |
|19,400,000 |
||
|- |
|- |
||
| |
| 26 |
||
| 58 |
| 58 |
||
| [[cerium]] |
| [[cerium]] |
||
| Line 337: | Line 326: | ||
| |
| |
||
|- |
|- |
||
| |
| 27 |
||
| 60 |
| 60 |
||
| [[neodymium]] |
| [[neodymium]] |
||
| Line 348: | Line 337: | ||
| |
| |
||
|- |
|- |
||
| |
| 28 |
||
| 57 |
| 57 |
||
| [[lanthanum]] |
| [[lanthanum]] |
||
| Line 359: | Line 348: | ||
| |
| |
||
|- |
|- |
||
| |
| 29 |
||
| 39 |
| 39 |
||
| [[yttrium]] |
| [[yttrium]] |
||
| Line 370: | Line 359: | ||
|6,000 |
|6,000 |
||
|- |
|- |
||
| |
| 30 |
||
| 7 |
| 7 |
||
| [[nitrogen]] |
| [[nitrogen]] |
||
| Line 381: | Line 370: | ||
|140,000,000 |
|140,000,000 |
||
|- |
|- |
||
| |
| 31 |
||
| 27 |
| 27 |
||
| [[cobalt]] |
| [[cobalt]] |
||
| Line 392: | Line 381: | ||
|123,000 |
|123,000 |
||
|- |
|- |
||
| |
| 32 |
||
| 3 |
| 3 |
||
| [[lithium]] |
| [[lithium]] |
||
| Line 403: | Line 392: | ||
|35,000 |
|35,000 |
||
|- |
|- |
||
| |
| 33 |
||
| 41 |
| 41 |
||
| [[niobium]] |
| [[niobium]] |
||
| Line 414: | Line 403: | ||
|64,000 |
|64,000 |
||
|- |
|- |
||
| |
| 34 |
||
| 31 |
| 31 |
||
| [[gallium]] |
| [[gallium]] |
||
| Line 425: | Line 414: | ||
| |
| |
||
|- |
|- |
||
| |
| 35 |
||
| 21 |
| 21 |
||
| [[scandium]] |
| [[scandium]] |
||
| Line 436: | Line 425: | ||
| |
| |
||
|- |
|- |
||
| |
| 36 |
||
| 82 |
| 82 |
||
| [[lead]] |
| [[lead]] |
||
| Line 447: | Line 436: | ||
|4,820,000 |
|4,820,000 |
||
|- |
|- |
||
| |
| 37 |
||
| 62 |
| 62 |
||
| [[samarium]] |
| [[samarium]] |
||
| Line 458: | Line 447: | ||
| |
| |
||
|- |
|- |
||
| |
| 38 |
||
| 90 |
| 90 |
||
| [[thorium]] |
| [[thorium]] |
||
| Line 469: | Line 458: | ||
| |
| |
||
|- |
|- |
||
| |
| 39 |
||
| 59 |
| 59 |
||
| [[praseodymium]] |
| [[praseodymium]] |
||
| Line 480: | Line 469: | ||
| |
| |
||
|- |
|- |
||
| |
| 40 |
||
| 5 |
| 5 |
||
| [[boron]] |
| [[boron]] |
||
| B |
| B |
||
| |
| |
||
| 950 |
| 950 {{dubious}} |
||
| 8.7 |
| 8.7 |
||
| |
| |
||
| Line 491: | Line 480: | ||
|9,400,000 |
|9,400,000 |
||
|- |
|- |
||
| |
| 41 |
||
| 64 |
| 64 |
||
| [[gadolinium]] |
| [[gadolinium]] |
||
| Line 502: | Line 491: | ||
| |
| |
||
|- |
|- |
||
| |
| 42 |
||
| 66 |
| 66 |
||
| [[dysprosium]] |
| [[dysprosium]] |
||
| Line 513: | Line 502: | ||
| |
| |
||
|- |
|- |
||
| |
| 43 |
||
| 72 |
| 72 |
||
| [[hafnium]] |
| [[hafnium]] |
||
| Line 524: | Line 513: | ||
| |
| |
||
|- |
|- |
||
| |
| 44 |
||
| 68 |
| 68 |
||
| [[erbium]] |
| [[erbium]] |
||
| Line 535: | Line 524: | ||
| |
| |
||
|- |
|- |
||
| |
| 45 |
||
| 70 |
| 70 |
||
| [[ytterbium]] |
| [[ytterbium]] |
||
| Line 546: | Line 535: | ||
| |
| |
||
|- |
|- |
||
| |
| 46 |
||
| 55 |
| 55 |
||
| [[caesium]] |
| [[caesium]] |
||
| Line 557: | Line 546: | ||
| |
| |
||
|- |
|- |
||
| |
| 47 |
||
| 4 |
| 4 |
||
| [[beryllium]] |
| [[beryllium]] |
||
| Line 568: | Line 557: | ||
|220 |
|220 |
||
|- |
|- |
||
| |
| 48 |
||
| 50 |
| 50 |
||
| [[tin]] |
| [[tin]] |
||
| Line 579: | Line 568: | ||
|280,000 |
|280,000 |
||
|- |
|- |
||
| |
| 49 |
||
| 63 |
| 63 |
||
| [[europium]] |
| [[europium]] |
||
| Line 590: | Line 579: | ||
| |
| |
||
|- |
|- |
||
| |
| 50 |
||
| 92 |
| 92 |
||
| [[uranium]] |
| [[uranium]] |
||
| Line 601: | Line 590: | ||
|74,119 |
|74,119 |
||
|- |
|- |
||
| |
| 51 |
||
| 73 |
| 73 |
||
| [[tantalum]] |
| [[tantalum]] |
||
| Line 612: | Line 601: | ||
|1,100 |
|1,100 |
||
|- |
|- |
||
| |
| 52 |
||
| 32 |
| 32 |
||
| [[germanium]] |
| [[germanium]] |
||
| Line 622: | Line 611: | ||
| 1.5 |
| 1.5 |
||
|155 |
|155 |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| 160.6 {{dubious}} |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|- |
|- |
||
| 54 |
| 54 |
||
Revision as of 22:17, 29 May 2019
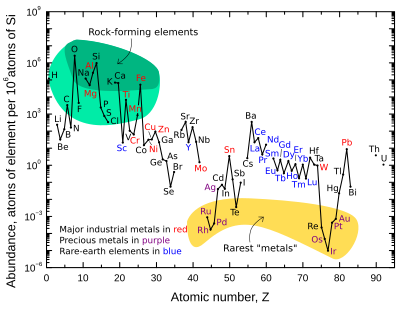
The abundance of elements in Earth's crust is shown in tabulated form with the estimated crustal abundance for each chemical element shown as parts per million (ppm) by mass (10,000 ppm = 1%). Note that the noble gases are not included, as they form no part of the solid crust. Also not included are certain elements with extremely low crustal concentrations: technetium (atomic number 43), promethium (61), and all elements with atomic numbers greater than 83 except thorium (90) and uranium (92).
| Rank | Z | Element | Symbol | Abundance in crust (ppm) by source | Annual production | ||||
|---|---|---|---|---|---|---|---|---|---|
| Darling[2] | Barbalace[3] | WebElements[4] | Israel Science and Technology[5] | Jefferson Lab[6] | (2016, tonnes)[7] | ||||
| 1 | 8 | oxygen | O | 466,000 | 474,000 | 460,000 | 467,100 | 461,000 | |
| 2 | 14 | silicon | Si | 277,200 | 277,100 | 270,000 | 276,900 | 282,000 | 7,200,000 |
| 3 | 13 | aluminium | Al | 81,300 | 82,000 | 82,000 | 80,700 | 82,300 | 57,600,000 |
| 4 | 26 | iron | Fe | 50,000 | 41,000 | 63,000 | 50,500 | 56,300 | 1,150,000,000 |
| 5 | 20 | calcium | Ca | 36,300 | 41,000 | 50,000 | 36,500 | 41,500 | |
| 6 | 11 | sodium | Na | 28,300 | 23,000 | 23,000 | 27,500 | 23,600 | 255,000,000 |
| 7 | 12 | magnesium | Mg | 20,900 | 23,000 | 29,000 | 20,800 | 23,300 | 1,010,000 |
| 8 | 19 | potassium | K | 25,900 | 21,000 | 15,000 | 25,800 | 20,900 | |
| 9 | 22 | titanium | Ti | 4,400 | 5,600 | 6,600 | 6,200 | 5,600 | 6,600,000 |
| 10 | 1 | hydrogen | H | 1,400 | 1,500 | 1,400 | 1,400 | ||
| 11 | 15 | phosphorus | P | 1,200 | 1,000 | 1,000 | 1,300 | 1,050 | |
| 12 | 25 | manganese | Mn | 1,000 | 950 | 1,100 | 900 | 950 | 16,000,000 |
| 13 | 9 | fluorine | F | 800 | 950 | 540 | 290 | 585 | |
| 14 | 56 | barium | Ba | 500 | 340 | 340 | 500 | 425 | |
| 15 | 6 | carbon | C | 300 | 480 | 1,800 | 940 | 200 | |
| 16 | 38 | strontium | Sr | 370 | 360 | 370 | 350,000 | ||
| 17 | 16 | sulfur | S | 500 | 260 | 420 | 520 | 350 | 69,300,000 |
| 18 | 40 | zirconium | Zr | 190 | 130 | 250 | 165 | 1,460,000 | |
| 19 | 23 | vanadium | V | 100 | 160 | 190 | 120 | 76,000 | |
| 20 | 17 | chlorine | Cl | 500 | 130 | 170 | 450 | 145 | |
| 21 | 24 | chromium | Cr | 100 | 100 | 140 | 350 | 102 | 26,000,000 |
| 22 | 37 | rubidium | Rb | 300 | 90 | 60 | 90 | ||
| 23 | 28 | nickel | Ni | 80 | 90 | 190 | 84 | 2,250,000 | |
| 24 | 30 | zinc | Zn | 75 | 79 | 70 | 11,900,000 | ||
| 25 | 29 | copper | Cu | 100 | 50 | 68 | 60 | 19,400,000 | |
| 26 | 58 | cerium | Ce | 68 | 60 | 66.5 | |||
| 27 | 60 | neodymium | Nd | 38 | 33 | 41.5 | |||
| 28 | 57 | lanthanum | La | 32 | 34 | 39 | |||
| 29 | 39 | yttrium | Y | 30 | 29 | 33 | 6,000 | ||
| 30 | 7 | nitrogen | N | 50 | 25 | 20 | 19 | 140,000,000 | |
| 31 | 27 | cobalt | Co | 20 | 30 | 25 | 123,000 | ||
| 32 | 3 | lithium | Li | 20 | 17 | 20 | 35,000 | ||
| 33 | 41 | niobium | Nb | 20 | 17 | 20 | 64,000 | ||
| 34 | 31 | gallium | Ga | 18 | 19 | 19 | |||
| 35 | 21 | scandium | Sc | 16 | 26 | 22 | |||
| 36 | 82 | lead | Pb | 14 | 10 | 14 | 4,820,000 | ||
| 37 | 62 | samarium | Sm | 7.9 | 6 | 7.05 | |||
| 38 | 90 | thorium | Th | 12 | 6 | 9.6 | |||
| 39 | 59 | praseodymium | Pr | 9.5 | 8.7 | 9.2 | |||
| 40 | 5 | boron | B | 950 [dubious – discuss] | 8.7 | 10 | 9,400,000 | ||
| 41 | 64 | gadolinium | Gd | 7.7 | 5.2 | 6.2 | |||
| 42 | 66 | dysprosium | Dy | 6 | 6.2 | 5.2 | |||
| 43 | 72 | hafnium | Hf | 5.3 | 3.3 | 3.0 | |||
| 44 | 68 | erbium | Er | 3.8 | 3.0 | 3.5 | |||
| 45 | 70 | ytterbium | Yb | 3.3 | 2.8 | 3.2 | |||
| 46 | 55 | caesium | Cs | 3 | 1.9 | 3 | |||
| 47 | 4 | beryllium | Be | 2.6 | 1.9 | 2.8 | 220 | ||
| 48 | 50 | tin | Sn | 0 | 2.2 | 2.2 | 2.3 | 280,000 | |
| 49 | 63 | europium | Eu | 2.1 | 1.8 | 2.0 | |||
| 50 | 92 | uranium | U | 0 | 1.8 | 2.7 | 74,119 | ||
| 51 | 73 | tantalum | Ta | 2 | 1.7 | 2.0 | 1,100 | ||
| 52 | 32 | germanium | Ge | 1.8 | 1.4 | 1.5 | 155 | ||
| 53 | 74 | tungsten | W | 160.6 [dubious – discuss] | 1.1 | 1.25 | 86,400 | ||
| 54 | 42 | molybdenum | Mo | 1.5 | 1.1 | 1.2 | 227,000 | ||
| 55 | 33 | arsenic | As | 1.5 | 2.1 | 1.8 | 36,500 | ||
| 56 | 67 | holmium | Ho | 1.4 | 1.2 | 1.3 | |||
| 57 | 65 | terbium | Tb | 1.1 | 0.9400 | 1.2 | |||
| 58 | 69 | thulium | Tm | 0.4800 | 0.4500 | 0.52 | |||
| 59 | 35 | bromine | Br | 0.3700 | 3 | 2.4 | 391,000 | ||
| 60 | 81 | thallium | Tl | 0.6000 | 0.5300 | 0.850 | 10 | ||
| 61 | 71 | lutetium[8] | Lu | 0.5 | |||||
| 62 | 51 | antimony | Sb | 0.2000 | 0.2000 | 0.2 | 130,000 | ||
| 63 | 53 | iodine | I | 0.1400 | 0.4900 | 0.450 | 31,600 | ||
| 64 | 48 | cadmium | Cd | 0.1100 | 0.1500 | 0.15 | 23,000 | ||
| 65 | 47 | silver | Ag | 0.0700 | 0.0800 | 0.075 | 27,000 | ||
| 66 | 80 | mercury | Hg | 0.0500 | 0.0670 | 0.085 | 4,500 | ||
| 67 | 34 | selenium | Se | 0.0500 | 0.0500 | 0.05 | 2,200 | ||
| 68 | 49 | indium | In | 0.0490 | 0.1600 | 0.250 | 655 | ||
| 69 | 83 | bismuth | Bi | 0.0480 | 0.0250 | 0.0085 | 10,200 | ||
| 70 | 52 | tellurium | Te | 0.0050 | 0.0010 | 0.001 | 2,200 | ||
| 71 | 78 | platinum | Pt | 0.0030 | 0.0037 | 0.005 | 172 | ||
| 72 | 79 | gold | Au | 0.0011 | 0.0031 | 0.004 | 3,100 | ||
| 73 | 44 | ruthenium | Ru | 0.0010 | 0.0010 | 0.001 | |||
| 74 | 46 | palladium | Pd | 0.0006 | 0.0063 | 0.015 | 208 | ||
| 75 | 75 | rhenium | Re | 0.0004 | 0.0026 | 0.0007 | 47.2 | ||
| 76 | 77 | iridium | Ir | 0.0003 | 0.0004 | 0.001 | |||
| 77 | 45 | rhodium | Rh | 0.0002 | 0.0007 | 0.001 | |||
| 78 | 76 | osmium | Os | 0.0001 | 0.0018 | 0.0015 | |||
See also
- Abundances of the elements (data page)
- Primordial nuclide
- List of chemical elements
- Atmospheric chemistry
References
- ^ Anderson, Don L.; ‘Chemical Composition of the Mantle’ in Theory of the Earth, pp. 147-175 ISBN 0865421234
- ^ "Elements, Terrestrial Abundance". www.daviddarling.info. Archived from the original on 10 April 2007. Retrieved 2007-04-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Barbalace, Kenneth. "Periodic Table of Elements". Environmental Chemistry.com. Retrieved 2007-04-14.
- ^
"Abundance in Earth's Crust". WebElements.com. Archived from the original on 9 March 2007. Retrieved 2007-04-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "List of Periodic Table Elements Sorted by Abundance in Earth's crust". Israel Science and Technology Homepage. Retrieved 2007-04-15.
- ^
"It's Elemental — The Periodic Table of Elements". Jefferson Lab. Archived from the original on 29 April 2007. Retrieved 2007-04-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Commodity Statistics and Information. USGS. All production numbers are for mines, except for Al, Cd, Fe, Ge, In, N, Se (plants, refineries), S (all forms) and As, Br, Mg, Si (unspecified). Data for B, K, Ti, Y are given not for the pure element but for the most common oxide, data for Na and Cl are for NaCl. For many elements like Si, Al, data are ambiguous (many forms produced) and are taken for the pure element. U data is pure element required for consumption by current reactor fleet [1]. WNA.
- ^ Emsley, John (2001). Nature's building blocks: an A-Z guide to the elements. Oxford University Press. pp. 240–242. ISBN 0-19-850341-5.
- BookRags, Periodic Table.
- World Book Encyclopedia, Exploring Earth.
- HyperPhysics, Georgia State University, Abundance of Elements in Earth's Crust.
- Data Series 140, Historical Statistics for Mineral and Material Commodities in the United States, Version 2011, USGS [2].
- Eric Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, 2007
