Hydrogen storage
Hydrogen storage describes the methodologies for storing H2 for subsequent use. The methodologies span many approaches, including high pressures, but usually focus on chemical compounds that reversibly release H2 upon heating. Hydrogen storage is a topical goal in the development of a hydrogen economy. Most research into hydrogen storage is focused on storing hydrogen in a lightweight, compact manner for mobile applications.
Some attention has been given to the role of hydrogen to provide grid energy storage for unpredictable energy sources, like wind power.
Hydrocarbons are stored extensively at the point of use, be it in the gasoline tanks of automobiles or propane tanks hung on the side of barbecue grills. Hydrogen, in comparison, is quite difficult to store or transport with current technology. Hydrogen gas has good energy density by weight, but poor energy density by volume versus hydrocarbons, hence it requires a larger tank to store. A large hydrogen tank will be heavier than the small hydrocarbon tank used to store the same amount of energy, all other factors remaining equal. Increasing gas pressure would improve the energy density by volume, making for smaller, but not lighter container tanks (see pressure vessel). Compressing a gas will require energy to power the compressor. Higher compression will mean more energy lost to the compression step.
Alternatively, higher volumetric energy density liquid hydrogen may be used (as in the Space Shuttle). However liquid hydrogen requires cryogenic storage and boils around 20.268 K (–252.882 °C or -423.188 °F). Hence, its liquefaction imposes a large energy loss, used to cool it down to that temperature. The tanks must also be well insulated to prevent boil off. Ice may form around the tank and help corrode it further if the insulation fails. Insulation for liquid hydrogen tanks is usually expensive and delicate. Assuming all of that is solvable, the density problem remains. Liquid hydrogen has worse energy density by volume than hydrocarbon fuels such as gasoline by approximately a factor of four. This highlights the density problem for pure hydrogen: there is actually about 64% more hydrogen in a liter of gasoline (116 grams hydrogen) than there is in a liter of pure liquid hydrogen (71 grams hydrogen). Additionally, the carbon in the gasoline contributes to the energy of combustion.
Mobile storage targets

Targets are set by the FreedomCAR Partnership in January of 2002 between the United States Council for Automotive Research (USCAR) and U.S. DOE (Targets assume a 5-kg H2 storage system). The 2005 targets were not reached.[1]
Proposals and research
Metal hydrides
Metal hydrides, with varying degrees of efficiency, can be used as a storage medium for hydrogen, often reversibly. Some are easy-to-fuel liquids at ambient temperature and pressure, others are solids which could be turned into pellets. Proposed hydrides for use in a hydrogen economy include simple hydrides of magnesium or transition metals and complex metal hydrides, typically containing sodium, lithium, or calcium and aluminium or boron. These materials have good energy density by volume, although their energy density by weight is often worse than the leading hydrocarbon fuels. Furthermore, high temperatures are often required to release their hydrogen content.
Solid hydride storage is a leading contender for automotive storage. A hydride tank is about three times larger and four times heavier than a gasoline tank holding the same energy. For a standard car, that's about 45 US gallons (0.17 m³) of space and 600 pounds (270 kg) versus 15 US gallons (0.057 m³) and 150 pounds (70 kg). A standard gasoline tank weighs a few dozen pounds (tens of kilograms) and is made of steel costing less than a dollar a pound ($2.20/kg). Lithium, the primary constituent by weight of a hydride storage vessel, currently costs over $40 a pound ($90/kg). Any hydride will need to be recycled or recharged with hydrogen, either on board the automobile or at a recycling plant. A metal-oxide fuel cell, (i.e. zinc-air fuel cell or lithium-air fuel cell), may provide a better use for the added weight, than a hydrogen fuel cell with a metal hydride storage tank.
Often hydrides react by combusting rather violently upon exposure to moist air, and are quite toxic to humans in contact with the skin or eyes, hence cumbersome to handle (see borane, lithium aluminum hydride). For this reason, such fuels, despite being proposed and vigorously researched by the space launch industry, have never been used in any actual launch vehicle.
Few hydrides provide low reactivity (high safety) and high hydrogen storage densities (above 10% by weight). Leading candidates are sodium borohydride, lithium aluminum hydride and ammonia borane. Sodium borohydride and ammonia borane can be stored as a liquid when mixed with water, but must be stored at very high concentrations to produce desirable hydrogen densities, thus requiring complicated water recycling systems in a fuel cell. As a liquid, sodium borohydride provides the advantage of being able to react directly in a fuel cell, allowing the production of cheaper, more efficient and more powerful fuels cells that do not need platinum catalysts. Recycling sodium borohydride is energy expensive and would require recycling plants. More energy efficient means of recycling sodium borohydride are still experimental. Recycling ammonia borane by any means is still experimental.
New Scientist [2] state that Arizona State University is investigating using a borohydride solution to store hydrogen, which is released when the solution flows over a catalyst made of ruthenium.
Hydrogen produced for metal hydride storage must be of a high purity. Contaminants alter the nascent hydride surface and prevent absorption. This limits contaminants to at most 10 ppm oxygen in the hydrogen stream, with carbon monoxide, hydrocarbons and water at very low levels.
Synthesized hydrocarbons
An alternative to hydrides is to use regular hydrocarbon fuels as the hydrogen carrier. Then a small hydrogen reformer would extract the hydrogen as needed by the fuel cell. However, these reformers are slow to react to changes in demand and add a large incremental cost to the vehicle powertrain.
Direct methanol fuel cells do not require a reformer, but provide a lower energy density compared to conventional fuel cells, although this could be counter balanced with the much better energy densities of ethanol and methanol over hydrogen. Alcohol fuel is a renewable resource.
Solid-oxide fuel cells can operate on light hydrocarbons such as propane and methane without a reformer, or can run on higher hydrocarbons with only partial reforming, but the high temperature and slow startup time of these fuel cells are problematic for automotive applications.
Carbon nanotubes

Hydrogen carriers based on nanostructured carbon (such as carbon buckyballs and nanotubes) have been proposed. Despite initial claims of greater than 50 wt% hydrogen storage, it has generally come to be accepted that less than 1 wt% is practical.[3]
Metal-organic frameworks
Metal-organic frameworks represent another class of synthetic porous materials that store hydrogen. In 2006, chemists at UCLA and the University of Michigan have achieved hydrogen storage concentrations of up to 7.5% weight in a metal-organic framework material. However, the storage was achieved at the low temperature of 77 K. [4]
Ammonia
Ammonia (NH3) releases H2 in an appropriate catalytic reformer. Ammonia provides high hydrogen storage densities as a liquid with mild pressurization and cryogenic constraints: It can also be stored as a liquid at room temperature and pressure when mixed with water. Ammonia is the second most commonly produced chemical in the world and a large infrastructure for making, transporting, and distributing ammonia exists. Ammonia can be reformed to produce hydrogen with no harmful waste, or can mix with existing fuels and under the right conditions burn efficiently. Pure ammonia burns poorly at the atmospheric pressures found in natural gas fired water heaters and stoves. Under compression in an automobile engine it is a suitable fuel for slightly modified gasoline engines. Ammonia is a toxic gas at normal temperature and pressure and has a potent odor.
In September 2005 chemists from the Technical University of Denmark announced a method of storing hydrogen in the form of ammonia saturated into a salt tablet. They claim it will be an inexpensive and safe storage method. [5]
Amine borane complexes
Prior to 1980, several compounds have been investigated for hydrogen storage including complex borohydrides, or aluminohydrides, and ammonium salts. These hydrides have an upper theoretical hydrogen yield limited to about 8.5% by weight. Amongst the compounds that contain only B, N, and H (both positive and negative ions), representative examples include: amine boranes, boron hydride ammoniates, hydrazine-borane complexes, and ammonium octahydrotriborates or tetrahydroborates. Of these, amine boranes (and especially ammonia borane) have been extensively investigated as hydrogen carriers. During 1970's and 80's, the U.S. Army and Navy funded efforts aimed at developing hydrogen/deuterium gas-generating compounds for use in the HF/DF and HCl chemical lasers, and gas dynamic lasers. Earlier hydrogen gas-generating formulations used amine boranes and their derivatives. Ignition of the amine borane(s) forms boron nitride (BN) and hydrogen gas. In addition to ammonia borane (H3BNH3), other gas-generators include diborane diammoniate, H2B(NH3)2BH4.
Imidazolium ionic liquids
In 2007 Dupont and others reported hydrogen-storage materials based on imidazolium ionic liquids. Simple alkyl(aryl)-3-methylimidazolium N-bis(trifluoromethanesulfonyl)imidate salts that possess very low vapour pressure, high density, and thermal stability and are not inflammable can add reversibly 6-12 hydrogen atoms in the presence of classical Pd/C or Ir0 nanoparticle catalysts and can be used as alternative materials for on-board hydrogen-storage devices. These salts can hold up to 30 g L-1 of hydrogen at atmospheric pressure, which is twice that compressed hydrogen gas can attain at 350 atm. [6]
Phosphonium borate
In 2006 researchers of University of Windsor reported on reversible hydrogen storage in a non-metal phosphonium borate [7] [8] [9]:
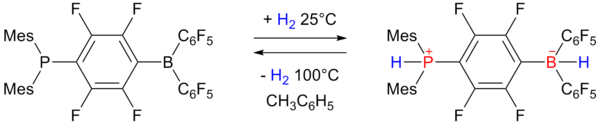
The phosphino-borane on the left accepts one equivalent of hydrogen at one atmosphere and 25°C and expels it again by heating to 100°C. The storage capacity is 0.25 wt% still rather below the 6 to 9 wt% required for practical use.
Doped polymers
In 2006 a team of Korean researchers led by Professor Lim Ji-sun of Seoul National University’s School of Physics proposed a new material with the hydrogen storage efficiency of 7.6 percent based on first-principles electronic structure calculations for hydrogen binding to metal-decorated polymers of many different kinds. According to these researchers, hydrogen can be stored in a solid material at ambient temperatures and pressures by attaching a titanium atom to a polyacetylene. [1][2]
Glass microspheres
Hollow glass microspheres can be utilized for controlled storage and release of hydrogen.
See also
External links
- United States Department of Energy Planned program activities for 2003-2010
- Hyweb (1996)
- Research into metal-organic framework or Nano Cages [3][4]
- Hydrogen Storage Technical Data
References
- ^ http://www.uscar.org/commands/files_download.php?files_id=82
- ^ "New type of hydrogen fuel cell powers up". newscientist. Retrieved 2006-09-16.
- ^ http://dx.doi.org/10.1016/j.jallcom.2006.11.192
- ^ http://www.greencarcongress.com/2006/03/researchers_dem.html
- ^ http://www.netpublikationer.dk/um/6567/html/chapter12.htm
- ^ Stracke, M. P. ; Ebeling, G. ; Cataluña, R. ; Dupont, J. Energy & Fuels 2007, 21, 1695-1698. doi:10.1021/ef060481t
- ^ Reversible, Metal-Free Hydrogen Activation Gregory C. Welch, Ronan R. San Juan, Jason D. Masuda, Douglas W. Stephan Science (journal) 17 November 2006: Vol. 314. no. 5802, pp. 1124 - 1126 doi:10.1126/science.1134230
- ^ H2 Activation, Reversibly Metal-free compound readily breaks and makes hydrogen Elizabeth Wilson Chemical & Engineering News November 20, 2006 Link
- ^ Mes stands for a mesityl substituent and C6F5 for a pentafluorophenyl group, see also tris(pentafluorophenyl)boron

