Gamma ray

| Nuclear physics |
|---|
 |
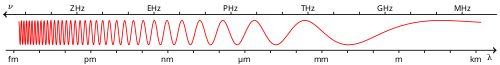
Gamma rays (denoted as γ) are electromagnetic radiation of high energy. They are produced by sub-atomic particle interactions, such as electron-positron annihilation, neutral pion decay, radioactive decay, fusion, fission or inverse Compton scattering in astrophysical processes. Gamma rays typically have frequencies above 1019 Hz and therefore energies above 100 keV and wavelength less than 10 picometers, often smaller than an atom. Gamma radioactive decay photons commonly have energies of a few hundred KeV, and are almost always less than 10 MeV in energy.
Because they are a form of ionizing radiation, gamma rays can cause serious damage when absorbed by living tissue, and are therefore a health hazard.
Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Alpha and beta "rays" had already been separated and named by the work of Ernest Rutherford in 1899, and in 1903 Rutherford named Villard's distinct new radiation "gamma rays."
In the past, the distinction between X-rays and gamma rays was based on energy (or equivalently frequency or wavelength), the latter being considered a higher-energy version of the former. However, high-energy X-rays produced by linear accelerators ("linacs") and astrophysical processes now often have higher energy than gamma rays produced by radioactive gamma decay. (In fact, one of the most common gamma-ray emitting isotopes used in nuclear medicine, technetium-99m produces gamma radiation of about the same energy (140 kev) as produced by a diagnostic X-ray machine, and significantly lower energy than the therapeutic treatment X-rays produced by linac machines in cancer radiotherapy.) Because of this overlap in energy ranges, the two types of electromagnetic radiation are now usually defined by their origin: X-rays are emitted by electrons outside the nucleus (and when produced by therapeutic linacs are often simply called "photons"), while gamma rays are specifically emitted by the nucleus (that is, produced by gamma decay). In theory, there is no lower limit to the energy of such photons, and thus "ultraviolet gamma rays" have been postulated. In certain fields such as astronomy, gamma rays and X-rays are still sometimes defined by energy, as the processes which produce them may be uncertain.
Properties
Shielding
Shielding gamma rays requires large amounts of mass. They are better absorbed by materials with high atomic numbers and high density, although neither effect is important compared to the total mass per area in the path of the gamma ray. For this reason, a lead shield is only modestly better (20-30%) as a gamma shield than an equal weight of another shielding material such as aluminum, concrete, or soil; the lead's major advantage is in its compactness.
The higher the energy of the gamma rays, the thicker the shielding required. Materials for shielding gamma rays are typically measured by the thickness required to reduce the intensity of the gamma rays by one half (the half value layer or HVL). For example, gamma rays that require 1 cm (0.4") of lead to reduce their intensity by 50% will also have their intensity reduced in half by 4.1 cm of Granite rock, 6 cm (2½") of concrete or 9 cm (3½") of packed soil. However, (again) the mass of this much concrete or soil is only 20-30% larger than that of this amount of lead. Depleted uranium is used for shielding in portable gamma ray sources, but again the savings in weight over lead is modest, and the main effect is to reduce shielding bulk.
Matter interaction


When a gamma ray passes through matter, the probability for absorption in a thin layer is proportional to the thickness of that layer. This leads to an exponential decrease of intensity with thickness. The exponential absorption holds only for a narrow beam of gamma rays. If a wide beam of gamma rays passes through a thick slab of concrete, the scattering from the sides reduces the absorption.
Here, μ = nσ is the absorption coefficient, measured in cm−1, n the number of atoms per cm3 in the material, σ the absorption cross section in cm2 and d the thickness of material in cm.
In passing through matter, gamma radiation ionizes via three main processes: the photoelectric effect, Compton scattering, and pair production.
- Photoelectric effect: This describes the case in which a gamma photon interacts with and transfers its energy to an atomic electron, ejecting that electron from the atom. The kinetic energy of the resulting photoelectron is equal to the energy of the incident gamma photon minus the binding energy of the electron. The photoelectric effect is the dominant energy transfer mechanism for x-ray and gamma ray photons with energies below 50 keV (thousand electron volts), but it is much less important at higher energies.
- Compton scattering: This is an interaction in which an incident gamma photon loses enough energy to an atomic electron to cause its ejection, with the remainder of the original photon's energy being emitted as a new, lower energy gamma photon with an emission direction different from that of the incident gamma photon. The probability of Compton scatter decreases with increasing photon energy. Compton scattering is thought to be the principal absorption mechanism for gamma rays in the intermediate energy range 100 keV to 10 MeV. Compton scattering is relatively independent of the atomic number of the absorbing material, which is why very dense metals like lead are only modestly better shields, on a per weight basis, than are less dense materials (as mentioned previously).
- Pair production: This becomes possible with gamma energies exceeding 1.02 MeV, and becomes important as an absorption mechanism at energies over about 5 MeV (see illustration at right, for lead). By interaction with the electric field of a nucleus, the energy of the incident photon is converted into the mass of an electron-positron pair. Any gamma energy in excess of the equivalent rest mass of the two particles (1.02 MeV) appears as the kinetic energy of the pair and the recoil nucleus. At the end of the positron's range, it combines with a free electron. The entire mass of these two particles is then converted into two gamma photons of at least 0.51 MeV energy each (or higher according to the kinetic energy of the annihilated particles).
The secondary electrons (and/or positrons) produced in any of these three processes frequently have enough energy to produce much ionization themselves.
Light interaction
High-energy (from 80 to 500 GeV) gamma rays arriving from far far-distant quasars are used to estimate the extragalactic background light in the universe: The highest-energy rays interact more readily with the background light photons and thus their density may be estimated by analyzing the incoming gamma-ray spectrums.[1]
Gamma ray production
Gamma rays are often produced alongside other forms of radiation such as alpha or beta. When a nucleus emits an
α
or
β
particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower level by emitting a gamma ray in much the same way that an atomic electron can jump to a lower level by emitting visible light or ultraviolet radiation.

Co
Gamma rays, x-rays, visible light, and radio waves are all forms of electromagnetic radiation. The only difference is the frequency and hence the energy of the photons. Gamma rays are the most energetic. An example of gamma ray production follows.
First The element Link does not exist. decays to excited The element Link does not exist. by beta decay. Then the 60
Ni
drops down to the ground state (see nuclear shell model) by emitting two gamma rays in succession (1.17 MeV then 1.33 MeV):
The element Link does not exist. → The element Link does not exist. +
e−
+
ν
e+
γ
+ 1.17 MeV The element Link does not exist. → The element Link does not exist. +
γ
+ 1.33 MeV
Another example is the alpha decay of The element Link does not exist. to form The element Link does not exist.; this alpha decay is accompanied by gamma emission. In some cases, the gamma emission spectrum for a nucleus (daughter nucleus) is quite simple, (eg 60
Co
/60
Ni
) while in other cases, such as with (241
Am
/237
Np
and The element Link does not exist./192
Pt
), the gamma emission spectrum is complex, revealing that a series of nuclear energy levels can exist. The fact that an alpha spectrum can have a series of different peaks with different energies reinforces the idea that several nuclear energy levels are possible.

Because a beta decay is accompanied by the emission of a neutrino which also carries energy away, the beta spectrum does not have sharp lines, but instead is a broad peak. Hence from beta decay alone it is not possible to probe the different energy levels found in the nucleus.
In optical spectroscopy, it is well known that an entity which emits light can also absorb light at the same wavelength (photon energy). For instance, a sodium flame can emit yellow light as well as absorb the yellow light from a sodium vapor lamp. In the case of gamma rays, this can be seen in Mössbauer spectroscopy. Here, a correction for the energy lost by the recoil of the nucleus is made and the exact conditions for gamma ray absorption through resonance can be attained.
This is similar to the Franck Condon effects seen in optical spectroscopy.
Health effects
Gamma rays compete with neutrons as the most dangerous form of ionizing radiation emitted by something such as a nuclear explosion because they are highly penetrating, highly energetic ionizing radiation. Gamma rays have the shortest wavelength of all waves in the electromagnetic spectrum, and therefore have the greatest ability to penetrate through any gap, even a subatomic one, in what might otherwise be an effective shield. The most biological damaging forms of gamma radiation occur in the gamma ray window, between 3 and 10 MeV. See cobalt-60.
Gamma-rays are not stopped by the skin. They can induce DNA alteration by effect of whole-body gamma-irradiation on localized beta-irradiation-induced skin reactions in mice.[2]
Uses

This property means that gamma radiation is often used to kill living organisms, in a process called irradiation. Applications of this include sterilizing medical equipment (as an alternative to autoclaves or chemical means), removing decay-causing bacteria from many foods or preventing fruit and vegetables from sprouting to maintain freshness and flavor.
Due to their tissue penetrating property, gamma rays/X-rays have a wide variety of medical uses such as in CT Scans and radiation therapy (see X-ray). However, as a form of ionizing radiation they have the ability to effect molecular changes, giving them the potential to cause cancer when DNA is affected. The molecular changes can also be used to alter the properties of semi-precious stones, and is often used to change white topaz into blue topaz.
Despite their cancer-causing properties, gamma rays are also used to treat some types of cancer. In the procedure called gamma-knife surgery, multiple concentrated beams of gamma rays are directed on the growth in order to kill the cancerous cells. The beams are aimed from different angles to focus the radiation on the growth while minimizing damage to the surrounding tissues. (As an illustration of the radiation origin-process contributing to its name, a similar technique which uses photons from linacs rather than cobalt gamma decay, is called "Cyberknife").
Gamma rays are also used for diagnostic purposes in nuclear medicine. Several gamma-emitting radioisotopes are used, one of which is technetium-99m. When administered to a patient, a gamma camera can be used to form an image of the radioisotope's distribution by detecting the gamma radiation emitted. Such a technique can be employed to diagnose a wide range of conditions (e.g. spread of cancer to the bones).
In the US, gamma ray detectors are beginning to be used as part of the Container Security Initiative (CSI). These US$5 million machines are advertised to scan 30 containers per hour. The objective of this technique is to screen merchant ship containers before they enter US ports.
Body response
After gamma-irradiation, and the breaking of DNA double-strands, a cell can repair the damaged genetic material to the limit of its capability. However, a study of Rothkamm and Lobrich has shown that the repairing process works well after high-dose exposure but is much slower in the case of a low-dose exposure.[3]
This could mean that a chronic low-dose exposure cannot be fought by the body [citation needed]. The probability of detecting small alterations or of a detectable defect occurring is most likely small enough that the cell would replicate before initiating a full repair [citation needed]. Some cells cannot detect their own genetic defects [citation needed].
Risk assessment
The natural outdoor exposure in Great Britain ranges from 2 × 10–7 to 4 × 10–7 cSv/h (centisieverts per hour).[4] Natural exposure to gamma rays is about 0.1 to 0.2 cSv per year, and the average total amount of radiation received in one year per inhabitant in the USA is 0.36 cSv.[5]
By comparison, the radiation dose from chest radiography is a fraction of the annual naturally occurring background radiation dose,[6] and the dose from fluoroscopy of the stomach is, at most, 5 cSv on the skin of the back.
For acute full-body equivalent dose, 100 cSv causes slight blood changes; 200–350 cSv causes nausea, hair loss, hemorrhaging and will cause death in a sizable number of cases (10%–35%) without medical treatment; 500 cSv is considered approximately the LD50 (lethal dose for 50% of exposed population) for an acute exposure to radiation even with standard medical treatment; more than 500 cSv brings an increasing chance of death; eventually, above 750–1000 cSv, even extraordinary treatment, such as bone-marrow transplants, will not prevent the death of the individual exposed (see Radiation poisoning).[clarification needed][citation needed]
For low dose exposure, for example among nuclear workers, who receive an average yearly radiation dose of 1.9 cSv,[clarification needed] the risk of dying from cancer (excluding leukemia) increases by 2 percent. For a dose of 10 cSv, that risk increase is at 10 percent. By comparison, risk of dying from cancer was increased by 32 percent for the survivors of the atomic bombing of Hiroshima and Nagasaki.[7]
See also
- Radioactive decay
- Nuclear fission/fusion
- Annihilation
- Gamma spectroscopy
- Gamma-ray astronomy
- Gamma-ray burst
- Mössbauer effect
References
- ^ Bock, R. K. (2008-06-27). "Very-High-Energy Gamma Rays from a Distant Quasar: How Transparent Is the Universe?". Science. 320 (5884): pp 1752–1754. doi:10.1126/science.1157087. ISSN 0036-8075.
{{cite journal}}:|pages=has extra text (help); Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ International Journal of Radiation Biology, 1992; 62 (6): 729-733.
- ^ Rothkamm K. - Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses - Proceedings of the National Academy of Science of the USA, 2003; 100 (9) : 5057-5062.
- ^ Department for Environment, Food and Rural Affairs (Defra) UK – Keys facts about radioactivity – 2003, http://www.defra.gov.uk/environment/statistics/radioact/kf/rakf03.htm
- ^ United Nations Scientific Committee on the Effects of Atomic Radiation Annex E: Medical radiation exposures – Sources and Effects of Ionizing – 1993, p. 249, New York, UN
- ^ US National Council on Radiation Protection and Measurements – NCRP Report No. 93 – pp 53–55, 1987. Bethesda, Maryland, USA, NCRP
- ^ IARC – Cancer risk following low doses of ionizing radiation – a 15-country study – http://www.iarc.fr/ENG/Units/RCAa1.html
External links
- Basic reference on several types of radiation
- Radiation Q & A
- GCSE information
- Radiation information
- Gamma ray bursts
- The Lund/LBNL Nuclear Data Search - Contains information on gamma-ray energies from isotopes.
- Mapping soils with airborne detectors
 The LIVEChart of Nuclides - IAEA with filter on gamma-ray energy, in Java or HTML
The LIVEChart of Nuclides - IAEA with filter on gamma-ray energy, in Java or HTML- Health Physics Society Public Education Website