Aluminium chloride

| |

| |
| Names | |
|---|---|
| IUPAC names
aluminium trichloride
trichloroalumane trichloridoaluminium | |
| Other names
aluminum trichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.371 |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AlCl3 | |
| Molar mass | 133.34 g/mol (anhydrous) 241.43 g/mol (hexahydrate) |
| Appearance | white or pale yellow solid, hygroscopic |
| Density | 2.48 g/cm3 (anhydrous) 1.3 g/cm3 (hexahydrate) |
| Melting point | 192.4 °C *(anhydrous) 0 °C (hexahydrate) |
| Boiling point | 120 °C (hexahydrate) |
| 43.9 g/100 ml (0 °C) 44.9 g/100 ml (10 °C) 45.8 g/100 ml (20 °C) 46.6 g/100 ml (30 °C) 47.3 g/100 ml (40 °C) 48.1 g/100 ml (60 °C) 48.6 g/100 ml (80 °C) 49 g/100 ml (100 °C) | |
| Solubility | soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride slightly soluble in benzene |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Octahedral (solid) Tetrahedral (liquid) | |
| Trigonal planar (monomeric vapour) | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
anhydrous: 380 mg/kg, rat (oral) hexahydrate: 3311 mg/kg, rat (oral) |
| Related compounds | |
Other anions
|
Aluminium fluoride Aluminium bromide Aluminium iodide |
Other cations
|
Boron trichloride Gallium trichloride Indium(III) chloride Thallium(III) chloride Magnesium chloride |
| Supplementary data page | |
| Aluminium chloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aluminum chloride (AlCl3) is a compound of aluminium and chlorine. Pure samples are white, but it is often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point, and is ionicly bonded with covalent character. It sublimes at 178 °C. Molten AlCl3 conducts electricity poorly,[1] unlike more ionic halides such as sodium chloride. It exists in the solid state as a six-coordinate layer lattice.
AlCl3 adopts the "YCl3" structure, featuring Al3+ cubic close packed layered structure.[2] In contrast, AlBr3 has a more molecular structure, with the Al3+ centers occupying adjacent tetrahedral holes of the close-packed framework of Br− ions. In the melt, aluminium trichloride exists as the dimer Al2Cl6, with tetracoordinate aluminium. This is why the density of the liquid phase is so much less than the solid (1.78 g/cm3 vs 2.48 g/cm3. Al2Cl6 dimers are also found in the vapour phase. At higher temperatures this Al2Cl6 dimer dissociates into trigonal planar AlCl3, which is structurally analogous to BF3.
Aluminum chloride is highly deliquescent, and can explode upon abrupt contact with water because of the high heat of hydration. Aqueous solutions of AlCl3 are ionic and thus conduct electricity well. Such solutions are found to be acidic, indicative of partial hydrolysis of the Al3+ ion. The reactions can be described (simplified) as:
- [Al(H2O)6]3+ + H2O ⇌ [Al(OH)(H2O)5]2+ + H3O+
Aluminum chloride crystallizes from water as the hexahydrate AlCl3·6H2O, which has been used as a topical antiperspirant.
AlCl3 is probably the most commonly used Lewis acid and also one of the most powerful. It finds widespread application in the chemical industry as the classic catalyst for Friedel-Crafts reactions, both acylations and alkylations. It also finds use in polymerization and isomerization reactions of hydrocarbons.
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapor phase.[1]
Chemical properties
Aluminium chloride is a powerful Lewis acid, capable of forming stable Lewis acid-base adducts with even weak Lewis bases such as benzophenone or mesitylene.[3] Not surprisingly it forms AlCl4− in the presence of chloride ions.
In water, partial hydrolysis forms HCl gas or H3O+, as described in the overview above. Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions, giving a gelatinous precipitate of aluminium hydroxide upon reaction with the correct quantity of aqueous sodium hydroxide:
Preparation
Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures between 650 to 750 °C.[1]
- 2 Al + 3 Cl2 → 2 AlCl3
- 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Hydrated forms are prepared by dissolving aluminium oxides with dry hydrochloric acid at 150°C.
Uses
The Friedel-Crafts reaction[3] is the major use for aluminium chloride, for example in the preparation of anthraquinone (for the dyestuffs industry) from benzene and phosgene.[1] In the general Friedel-Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:[3]
With benzene derivatives, the major product is the para isomer. The alkylation reaction has many associated problems, such as in Friedel-Crafts, so it is less widely used than the acylation reaction. For both reactions, the aluminium chloride, as well as other materials and the equipment, must be moderately dry, although a trace of moisture is necessary for the reaction to proceed. A general problem with the Friedel-Crafts reaction is that the aluminium chloride "catalyst" needs to be present in full stoichiometric quantities in order for the reaction to go to completion, because it complexes strongly with the products (see chemical properties above). This makes it very difficult to recycle, so it must be destroyed after use, generating a large amount of corrosive waste. For this reason chemists are examining the use of more environmentally benign catalysts such as ytterbium(III) triflate or dysprosium(III) triflate, which can be recycled.
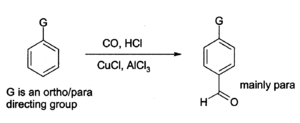
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst):[4]
Aluminium chloride finds a wide variety of other applications in organic chemistry.[5] For example, it can catalyse the "ene reaction", such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:[6]
AlCl3 is also widely used for polymerization and isomerization reactions of hydrocarbons. Important examples[1] include the manufacture of ethylbenzene, which used to make styrene and thus polystyrene, and also production of dodecylbenzene, which is used for making detergents.
Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis.
Aluminium chloride, often in the form of derivatives such as aluminium chlorohydrate, is a common component in antiperspirants at low concentrations. Hyperhidrosis sufferers need a much higher concentration (15% or higher), sold under such brand names as Drysol, Maxim, Odaban, CertainDri, B+Drier, Anhydrol Forte and Driclor.
Chemical reactions
Aluminium chloride reacts with calcium and magnesium hydrides in tetrahydrofuran forming tetrahydro aluminates:
- AlCl3 + 3 LiH → AlH3 + 3 LiCl
The hexahydrate decomposes to aluminum oxide when heated to 300 °C: [7]
- AlCl3 + 3H2O → Al(OH)3 + 3 HCl
Precautions
Anhydrous AlCl3 reacts vigorously with water and bases, so suitable precautions are required. Hydrated salts are less problematic. It can cause irritation to the eyes skin and the respiratory system if inhaled or on contact.[8]
References
- ^ a b c d e N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Pergamon Press, Oxford, United Kingdom, 1984.
- ^ A. F. Wells, Structural Inorganic Chemistry, Oxford Press, Oxford, United Kingdom, 1984.
- ^ a b c G. A. Olah (ed.), Friedel-Crafts and Related Reactions, Vol. 1, Interscience, New York, 1963.
- ^ L. G. Wade, Organic Chemistry, 5th edition, Prentice Hall, Upper Saddle River, New Jersey, United States, 2003.
- ^ P. Galatsis, in: Handbook of Reagents for Organic Synthesis: Acidic and Basic Reagents, (H. J. Reich, J. H. Rigby, eds.), pp. 12–15, Wiley, New York, 1999.
- ^ B. B. Snider (1980). "Lewis-acid catalyzed ene reactions". Acc. Chem. Res. 13: 426. doi:10.1021/ar50155a007.
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ http://www.solvaychemicals.us/static/wma/pdf/5/1/1/8/ALCL.pdf