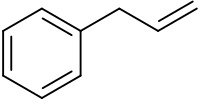
Allylbenzene
| |
| Names | |
|---|---|
| IUPAC name
Prop-2-enylbenzene
| |
| Other names
Allylbenzene; 3-Phenyl-1-propene; 2-Propenylbenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.005.542 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H10 | |
| Molar mass | 118.179 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.893 g/cm3 |
| Melting point | −40 °C (−40 °F; 233 K) |
| Boiling point | 156 °C (313 °F; 429 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phenylpropene is the organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to allyl. Phenylpropene isomerizes to trans-propenylbenzene.[1]
In plant biochemistry, the phenylpropene skeleton is the parent (simplest representation) of the phenylpropanoids. Prominent derivatives include eugenol, safrole, and many others.[2]
References
- ^ "Isomerization of Allylbenzenes". Chemical Reviews. 115: 5462–5569. 2015. doi:10.1021/acs.chemrev.5b00052.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Vogt, Thomas (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3 (1): 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
External links
 Media related to Allylbenzenes at Wikimedia Commons
Media related to Allylbenzenes at Wikimedia Commons
