ORG-25935
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
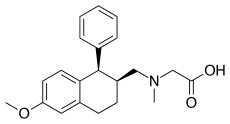
| Formula | C21H25NO3 |
| Molar mass | 339.435 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
ORG-25935, also known as SCH-900435 is a synthetic drug developed by Organon International, which acts as a selective inhibitor of the glycine transporter GlyT-1. In animal tests it reduces alcohol consumption and has analgesic and anticonvulsant effects, but it has mainly been studied for its antipsychotic properties, and in human trials it was shown to effectively counteract the effects of the dissociative drug ketamine.[1][2][3][4][5][6][7][8]
See also
References
- ^ Molander A, Lidö HH, Löf E, Ericson M, Söderpalm B (2006). "The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats". Alcohol and Alcoholism. 42 (1): 11–8. doi:10.1093/alcalc/agl085. PMID 17098748.
- ^ Morita K, Motoyama N, Kitayama T, Morioka N, Dohi T (December 2007). "Antinociceptive effects of glycine transporter inhibitors in neuropathic pain models in mice". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 130 (6): 458–63. doi:10.1254/fpj.130.458. PMID 18079595.
- ^ Lidö HH, Stomberg R, Fagerberg A, Ericson M, Söderpalm B (July 2009). "The glycine reuptake inhibitor org 25935 interacts with basal and ethanol-induced dopamine release in rat nucleus accumbens". Alcoholism, Clinical and Experimental Research. 33 (7): 1151–7. doi:10.1111/j.1530-0277.2009.00938.x. PMID 19389199.
- ^ Kalinichev M, Starr KR, Teague S, Bradford AM, Porter RA, Herdon HJ (May 2010). "Glycine transporter 1 (GlyT1) inhibitors exhibit anticonvulsant properties in the rat maximal electroshock threshold (MEST) test". Brain Research. 1331: 105–13. doi:10.1016/j.brainres.2010.03.032. PMID 20303337.
- ^ Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R (October 2010). "Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats". Biological Psychiatry. 68 (8): 704–11. doi:10.1016/j.biopsych.2010.05.029. PMID 20655511.
- ^ Musante V, Summa M, Cunha RA, Raiteri M, Pittaluga A (May 2011). "Pre-synaptic glycine GlyT1 transporter--NMDA receptor interaction: relevance to NMDA autoreceptor activation in the presence of Mg2+ ions". Journal of Neurochemistry. 117 (3): 516–27. doi:10.1111/j.1471-4159.2011.07223.x. PMID 21348870.
- ^ Jardemark K, Marcus MM, Malmerfelt A, Shahid M, Svensson TH (May 2012). "Differential effects of AMPA receptor potentiators and glycine reuptake inhibitors on antipsychotic efficacy and prefrontal glutamatergic transmission". Psychopharmacology. 221 (1): 115–31. doi:10.1007/s00213-011-2554-3. PMID 22068461.
- ^ D'Souza DC, Singh N, Elander J, Carbuto M, Pittman B, Udo de Haes J, et al. (March 2012). "Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence". Neuropsychopharmacology. 37 (4): 1036–46. doi:10.1038/npp.2011.295. PMC 3280648. PMID 22113087.
