Diborane

| |

| |
| Names | |
|---|---|
| IUPAC name
Diborane(6)
| |
| Other names
Boroethane
Boron hydride Diboron hexahydride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.039.021 |
| EC Number |
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| B2H6 | |
| Molar mass | 27.67 g·mol−1 |
| Appearance | Colorless gas |
| Odor | repulsive and sweet |
| Density | 1.216 g/L |
| Melting point | −164.85 °C (−264.73 °F; 108.30 K) |
| Boiling point | −92.5 °C (−134.5 °F; 180.7 K) |
| Reacts[1] | |
| Vapor pressure | 39.5 atm (16.6°C)[1] |
| Structure | |
| Tetrahedral (for boron) | |
| see text | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
highly flammable, reacts with water |
| NFPA 704 (fire diamond) | |
| 38 °C (100 °F; 311 K) | |
| Explosive limits | 0.8%-88%[1] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
40 ppm (rat, 4 hr) 29 ppm (mouse, 4 hr) 40-80 ppm (rat, 4 hr) 159-181 ppm (rat, 15 min)[2] |
LCLo (lowest published)
|
125 ppm (dog, 2 hr) 50 ppm (hamster, 8 hr)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.1 ppm (0.1 mg/m3)[1] |
REL (Recommended)
|
TWA 0.1 ppm (0.1 mg/m3)[1] |
IDLH (Immediate danger)
|
15 ppm[1] |
| Related compounds | |
Related boron compounds
|
Decaborane BF3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless and highly unstable gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature. Synonyms include boroethane, boron hydride, and diboron hexahydride.
Diborane is a key boron compound with a variety of applications. The compound is classified as "endothermic", meaning that its heat of formation, ΔH°f is positive (36 kJ/mol). Despite a high thermodynamic instability, diborane is surprisingly nonreactive for kinetic reasons, and it is known to take part in an extensive range of chemical transformations, many of them entailing loss of dihydrogen.
Structure and bonding
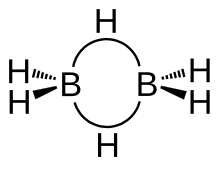
Diborane adopts a D2h structure containing four terminal and two bridging hydrogen atoms. The model determined by molecular orbital theory indicates that the bonds between boron and the terminal hydrogen atoms are conventional 2-center, 2-electron covalent bonds. The bonding between the boron atoms and the bridging hydrogen atoms is, however, different from that in molecules such as hydrocarbons. Having used two electrons in bonding to the terminal hydrogen atoms, each boron has one valence electron remaining for additional bonding. The bridging hydrogen atoms provide one electron each. Thus the B2H2 ring is held together by four electrons, an example of 3-center 2-electron bonding. This type of bond is sometimes called a 'banana bond'. The lengths of the B-Hbridge bonds and the B-Hterminal bonds are 1.33 and 1.19 Å respectively, and this difference in the lengths of these bonds reflects the difference in their strengths, the B-Hbridge bonds being relatively weaker. The weakness of the B-Hbridge vs B-Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ~2100 and 2500 cm−1, respectively.[4] The structure is isoelectronic with C2H62+, which would arise from the diprotonation of the planar molecule ethene.[5] Diborane is one of many compounds with such unusual bonding.[6]
Of the other elements in Group IIIA, gallium is known to form a similar compound, digallane, Ga2H6. Aluminium forms a polymeric hydride, (AlH3)n, although unstable Al2H6 has been isolated in solid hydrogen and is isostructural with diborane.[7]
Production and synthesis
Extensive studies of diborane have led to the development of multiple syntheses. Most preparations entail reactions of hydride donors with boron halides or alkoxides. The industrial synthesis of diborane involves the reduction of BF3 by sodium hydride, lithium hydride or lithium aluminium hydride:[8]
- 8 BF3 + 6 LiH → B2H6 + 6 LiBF4
Two laboratory methods start from boron trichloride with lithium aluminium hydride or from boron trifluoride ether solution with sodium borohydride. Both methods result in as much as 30% yield:
- 4 BCl3 + 3 LiAlH4 → 2 B2H6 + 3 LiAlCl4
- 4 BF3 + 3 NaBH4 → 2 B2H6 + 3 NaBF4
Older methods entail the direct reaction of borohydride salts with a non-oxidizing acid, such as phosphoric acid or dilute sulfuric acid.
- 2 BH4− + 2 H+ → 2 H2 + B2H6
Similarly, oxidation of borohydride salts has been demonstrated and remains convenient for small scale preparations. For example, using iodine as an oxidizer:
- 2 NaBH
4 + I
2 → 2 NaI + B
2H
6 + H
2
Another small-scale synthesis uses potassium hydroborate and phosphoric acid as starting materials.[9]
Reactions
Diborane is a highly reactive and versatile reagent that has a large number of applications.[10] Its dominating reaction pattern involves formation of adducts with Lewis bases. Often such initial adducts proceed rapidly to give other products. It reacts with ammonia to form the diammoniate of diborane, DADB, with lesser quantities of ammonia borane depending on the conditions used. Diborane also reacts readily with alkynes to form substituted alkene products which will readily undergo further addition reactions.
Diborane reacts with water to form hydrogen and boric acid:
- B2H6 + 6 H2O → 2 B(OH)3 + 6 H2
Diborane also reacts with methanol to give hydrogen and trimethoxyborate ester:[11]
- B2H6 + 6 MeOH → 2 B(OMe)3 + 6 H2
Treating diborane with sodium amalgam gives NaBH4 and Na[B3H8][11] When diborane is treated with lithium hydride in diethyl ether, Lithium borohydride is formed:[11]
- B2H6 + 2 LiH → 2 LiBH4
Diborane reacts with anhydrous hydrogen chloride or hydrogen bromide gas to give a boron halohydride:[11]
- B2H6 + HX → B2H5X + H2 (X = Cl, Br)
Reacting diborane with carbon monoxide at 470K and 20bar gives H3BCO.[11]
Reagent in organic synthesis
Diborane and its variants are central organic synthesis reagents for hydroboration, whereby alkenes add across the B-H bonds to give trialkylboranes. Diborane is used as a reducing agent roughly complementary to the reactivity of lithium aluminium hydride. The compound readily reduces carboxylic acids to the corresponding alcohols, whereas ketones react only sluggishly.
History
Diborane was first synthesised in the 19th century by hydrolysis of metal borides, but it was never analysed. From 1912 to 1936, the major pioneer in the chemistry of boron hydrides, Alfred Stock, undertook his research that led to the methods for the synthesis and handling of the highly reactive, volatile, and often toxic boron hydrides. He proposed the first ethane-like structure of diborane.[12] Electron diffraction measurements by S. H. Bauer initially appeared to support his proposed structure.[13][14]
Because of a personal communication with L. Pauling (who supported the ethane-like structure), H. I. Schlessinger did not specifically discuss 3-center-2-electron bonding in his then classic review in the early 1940s.[15] The review does, however, discuss the C2v structure in some depth, "It is to be recognized that this formulation easily accounts for many of the chemical properties of diborane..."
In 1943 an undergraduate student at Balliol College, Oxford, H. Christopher Longuet-Higgins, published the currently accepted structure together with R. P. Bell.[16] This structure had already been described in 1921.[17][18][19] The years following the Longuet-Higgins/Bell proposal witnessed a colorful discussion about the correct structure. The debate ended with the electron diffraction measurement in 1951 by K. Hedberg and V. Schomaker, with the confirmation of the structure shown in the schemes on this page.[20]
William Nunn Lipscomb, Jr. further confirmed the molecular structure of boranes using X-ray crystallography in the 1950s, and developed theories to explain its bonding. Later, he applied the same methods to related problems, including the structure of carboranes on which he directed the research of future Nobel Prize winner Roald Hoffmann. Lipscomb himself received the Nobel Prize in Chemistry in 1976 for his efforts.
Other uses
Diborane has been suggested as a rocket propellant and experimentally fired[21] but not used in any in service rocket, as a rubber vulcaniser, as a catalyst for hydrocarbon polymerisation, as a flame-speed accelerator, and as a doping agent for the production of semiconductors. It is also an intermediate in the production of highly pure boron for semiconductor production. It is also used to coat the walls of tokamaks to reduce the amount of heavy metal impurities in the core plasma.
Safety
The toxic effects of diborane are primarily due to its irritant properties. Short-term exposure to diborane can cause a sensation of tightness of the chest, shortness of breath, cough, and wheezing. These signs and symptoms can occur immediately or be delayed for up to 24 hours. Skin and eye irritation can also occur. Studies in animals have shown that diborane causes the same type of effects observed in humans. [citation needed]
People exposed for a long time to low amounts of diborane have experienced respiratory irritation, seizures, fatigue, drowsiness, confusion, and occasional transient tremors.
References
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0183". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Diborane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "International Chemical Safety Cards - Diborane". CDC/NIOSH ICSC Database. NIOSH Education and Information Division. Retrieved 14 August 2015.
- ^ Cooper, C. B., III; Shriver, D. F.; Onaka, S., "Vibrational spectroscopy of hydride-bridged transition metal compounds", Adv. Chem. Ser. 1978, 167, 232.
- ^ Rasul, G.; Prakash, G. K. S.; Olah, G. A. (2005). "Comparative ab initio Study of the Structures and Stabilities of the Ethane Dication C2H62+ and Its Silicon Analogues Si2H62+ and CSiH62+". Journal of Physical Chemistry A. 109 (5): 798–801. doi:10.1021/jp0404652. PMID 16838949.
- ^ Laszlo, P. (2000). "A Diborane Story". Angewandte Chemie International Edition. 39 (12): 2071–2072. doi:10.1002/1521-3773(20000616)39:12<2071::AID-ANIE2071>3.0.CO;2-C. PMID 10941018.
- ^ Andrews, L.; Wang, X. (2003). "The Infrared Spectrum of Al2H6 in Solid Hydrogen". Science. 299 (5615): 2049–2052. doi:10.1126/science.1082456. PMID 12663923.
- ^ Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry Vol. 1, 2nd Ed. Newyork: Academic Press. p. 773. ISBN 978-0121266011.
- ^ Norman, A. D.; Jolly, W. L.; Saturnino, D.; Shore, S. G. (1968). "Diborane". Inorganic Syntheses. 11: 15–19. doi:10.1002/9780470132425.ch4.
- ^ Mikhailov, B. M. (1962). "The Chemistry of Diborane". Russian Chemical Reviews. 31 (4): 207–224. doi:10.1070/RC1962v031n04ABEH001281.
- ^ a b c d e Housecroft, C. E.; Sharpe, A. G. (2008). "Chapter 13: The Group 13 Elements". Inorganic Chemistry (3rd ed.). Pearson. p. 336. ISBN 978-0-13-175553-6.
- ^ Stock, A. (1933). The Hydrides of Boron and Silicon. New York: Cornell University Press.
- ^ Bauer, S. H. (1937). "The Structure of Diborane". Journal of the American Chemical Society. 59 (6): 1096–1103. doi:10.1021/ja01285a041.
- ^ Bauer, S. H. (1942). "Structures and Physical Properties of the Hydrides of Boron and of their Derivatives". Chemical Reviews. 31 (1): 43–75. doi:10.1021/cr60098a002.
- ^ Schlesinger, H. I.; Burg, A. B. (1942). "Recent Developments in the Chemistry of the Boron Hydrides". Chemical Reviews. 31 (1): 1–41. doi:10.1021/cr60098a001.
- ^ Longuet-Higgins, H. C.; Bell, R. P. (1943). "64. The Structure of the Boron Hydrides". Journal of the Chemical Society (Resumed). 1943: 250–255. doi:10.1039/JR9430000250.
- ^ Dilthey, W. (1921). "Über die Konstitution des Wassers" (pdf). Angewandte Chemie. 34 (95): 596. doi:10.1002/ange.19210349509.
- ^ Nekrassov, B. V. (1940). Journal of General Chemistry of the USSR. 10: 1021.
{{cite journal}}: Missing or empty|title=(help) - ^ Nekrassov, B. V. (1940). Journal of General Chemistry of the USSR. 10: 1056.
{{cite journal}}: Missing or empty|title=(help) - ^ Hedberg, K.; Schomaker, V. (1951). "A Reinvestigation of the Structures of Diborane and Ethane by Electron Diffraction". Journal of the American Chemical Society. 73 (4): 1482–1487. doi:10.1021/ja01148a022.
- ^ Bilstein, Roger. "Stages to Saturn". chapter 5: NASA Public Affairs Office. p. 133. Retrieved 14 November 2015.
{{cite web}}: CS1 maint: location (link)
Further reading
H. C. Brown (1975). Organic Synthesis via Boranes. New York: John Wiley. ISBN 0-471-11280-1.

