Dimercaprol
| |

| |
| Names | |
|---|---|
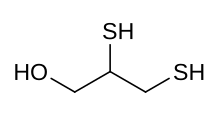
| IUPAC name
2,3-bis(sulfanyl)propan-1-ol
| |
| Other names
2,3-dimercaptopropanol
British anti-Lewisite | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 1732058 | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.394 |
| EC Number |
|
| KEGG | |
| MeSH | Dimercaprol |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2810 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C 3H 8S 2O | |
| Molar mass | 124.225 g mol−1 |
| Density | 1.239 g cm−3 |
| Boiling point | 120 °C; 248 °F; 393 K at 2.0 kPa |
| log P | 0.627 |
| Acidity (pKa) | 8.999 |
| Basicity (pKb) | 4.998 |
Refractive index (nD)
|
1.573 |
| Pharmacology | |
| V03AB09 (WHO) | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H301, H315, H319, H335 | |
| P261, P301+P310, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 112 °C (234 °F; 385 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimercaprol (INN), also called British anti-Lewisite or British antilewisite (abbreviated BAL), is a medication for chelation therapy in metal toxicity, including heavy metal poisoning. Dimercaprol is used medically in the treatment of toxic metal poisoning by arsenic, mercury, gold, lead, antimony, and some other metals.[1] In addition, it has in the past been used for the treatment of Wilson's disease, a genetic disorder in which the body tends to retain copper.[2]
Its original name reflects its origins as a compound secretly developed by British biochemists at Oxford University during World War II[3][4] as an antidote for lewisite, a now-obsolete arsenic-based chemical warfare agent.[3] Dimercaprol is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[5] Dimercaprol has long been the mainstay of chelation therapy for lead or arsenic poisoning,[6] and it remains an essential drug.[5] Nonetheless, because it can have serious adverse effects,[6] researchers have also pursued development of less toxic analogues.[6]
Biochemical function
Arsenic and some other heavy metals act by chemically reacting with adjacent thiol residues on metabolic enzymes, creating a chelate complex that inhibits the affected enzyme's activity.[7] Dimercaprol competes with the thiol groups for binding the metal ion, which is then excreted in the urine.[citation needed]
Dimercaprol is itself toxic, with a narrow therapeutic range and a tendency to concentrate arsenic in some organs. Other drawbacks include the need to administer it by painful intramuscular injection.[8] Serious side effects include nephrotoxicity and hypertension.
Dimercaprol has been found to form stable chelates in vivo with many other metals including inorganic mercury, antimony, bismuth, cadmium, chromium, cobalt, gold, and nickel. However, it is not necessarily the treatment of choice for toxicity to these metals. Dimercaprol has been used as an adjunct in the treatment of the acute encephalopathy of lead toxicity. It is a potentially toxic drug, and its use may be accompanied by multiple side effects. Although treatment with dimercaprol will increase the excretion of cadmium, there is a concomitant increase in renal cadmium concentration, so that its use in case of cadmium toxicity is to be avoided. It does, however, remove inorganic mercury from the kidneys; but is not useful in the treatment of alkylmercury or phenyl mercury toxicity. Dimercaprol also enhances the toxicity of selenium and tellurium, so it is not to be used to remove these elements from the body.[citation needed]
References
- ^ "Dimercaprol".
- ^ Denny-Brown D, PORTER H (December 1951). "The effect of BAL (2,3-dimercaptopropanol) on hepatolenticular degeneration (Wilson's disease)". N. Engl. J. Med. 245 (24): 917–25. doi:10.1056/NEJM195112132452401. PMID 14882450.
- ^ a b Domingo Tabangcura, Jr., G. Patrick Daubert. "British anti-Lewisite".
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Peters, R; Stocken, L; Thompson, R. (1945). "British Anti-Lewisite (BAL)". Nature. 156 (3969): 616–619. doi:10.1038/156616a0. PMID 21006485.
- ^ a b "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ a b c Flora, SJ; Pachauri, V (2010), "Chelation in metal intoxication", International Journal of Environmental Research and Public Health, 7 (7): 2745–2788, doi:10.3390/ijerph7072745, PMC 2922724, PMID 20717537.
{{citation}}: CS1 maint: unflagged free DOI (link) - ^ Goldman M, Dacre JC. (1989) Lewisite: its chemistry, toxicology, and biological effects. Rev Environ Contam Toxicol 110: 75-115
- ^ Mückter H, Liebl B, Reichl FX et al. (1997) Are we ready to replace dimercaprol (BAL) as an arsenic antidote? Human and Experimental Toxicology 16: 460-465

