Gephyrin
Gephyrin is a protein that in humans is encoded by the GPHN gene.[5][6][7][8][9]
This gene encodes a neuronal assembly protein that anchors inhibitory neurotransmitter receptors to the postsynaptic cytoskeleton via high affinity binding to a receptor subunit domain and tubulin dimers. In nonneuronal tissues, the encoded protein is also required for molybdenum cofactor biosynthesis. Mutations in this gene may be associated with the neurological condition hyperekplexia and also lead to molybdenum cofactor deficiency.
Gene
Numerous alternatively spliced transcript variants encoding different isoforms have been described; however, the full-length nature of all transcript variants is not currently known.[8] The production of alternatively spliced variants is affected by noncoding regions within the gene. A ‘yin-yang’ noncoding sequence pair encompassing gephyrin has been identified.[10] These sequences are opposites of each other - consisting of hundreds of divergent nucleotide states. Both of these patterns are uniquely human and evolved rapidly after splitting from their ancestral DNA pattern. The gephyrin yin and yang sequences are prevalent today in populations representing every major human ancestry.
Function
Gephyrin is a 93kDa multi-functional protein that is a component of the postsynaptic protein network of inhibitory synapses. It consists of 3 domains: N terminal G domain, C terminal E domain, and a large unstructured linker domain which connects the two. Although there are structures available for trimeric G and dimeric E domains, there is no structure available for the full length protein, which may be due to the large unstructured region which makes the protein hard to crystallize. But a recent study of the full length gephyrin by small-angle X-ray scattering shows that it predominantly forms trimers, and that because of its long linker region, it can exist in either a compact state or either of two extended states.[11]
Positive antibody staining for gephyrin at a synapse is most of the time consistent with the presence of glycine and/or GABAA receptors. Nevertheless, some exceptions can occur like in neurons of Dorsal Root Ganglions where gephyrin is absent despite the presence of GABAA receptors.[9] Gephyrin is considered a major scaffolding protein at inhibitory synapses, analogous in its function to that of PSD-95 at glutamatergic synapses.[12][13] Gephyrin was identified by its interaction with the glycine receptor, the main receptor protein of inhibitory synapses in the spinal cord and brainstem. In addition to its interaction with the glycine receptor, recent publications have shown that gephyrin also interacts with the intracellular loop between the transmembrane helices TM3 and TM4 of alpha and beta subunits of the GABAA receptor.[14]
Gephyrin displaces GABA receptors from the GABARAP/P130 complex, then brings the receptors to the synapse.[15] Once at the synapse, the protein binds to collybistin[16] and neuroligin 2.[17] In cells, gephyrin appears to form oligomers of at least three subunits. Several splice variants have been described that prevent this oligomerization without influencing the affinity for receptors. They nevertheless affect the composition of inhibitory synapses and can even play a role in diseases like epilepsy.[18]
The gephyrin protein is also required during molybdenum cofactor assembly for insertion of molybdenum.[19]
As aforementioned, gephyrin also catalyzes terminal two steps of Moco biosynthesis. In the penultimate step, N-terminal G domain adenylate the apo form of the molybdopterin to form the intermediate adenylated molybdopterin. In the terminal step, the C-terminal E domain catalyzes the deadenylation and also the metal insertion mechanism.
Clinical significance
Humans with temporal lobe epilepsy have been found to have abnormally low levels of gephyrin in their temporal lobes.[20] In animal models, a total lack of gephyrin results in stiff muscles and death immediately after birth. Stiff muscles are also a symptom of startle disease, that can be caused by a mutation in the gephyrin gene. And if a person produces auto-antibodies against gephyrin, this can even result in stiff person syndrome.[18]
Yin-yang sequences
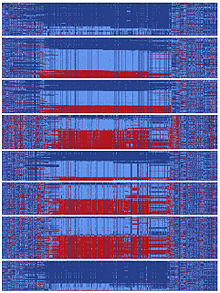
At some point in human history, there was a DNA sequence encompassing gephyrin that split and followed two divergent evolutionary paths.[10] These types of splits can occur when two populations become isolated from each other or when a chromosomal region does not experience recombination events. The two sequences that split from the ancestral sequence each acquired more than a hundred mutations that subsequently became common. Interestingly, this all happened in a relatively short time on an evolutionary scale, as hundreds of mutations were fixed in distinct ‘yin’ and ‘yang’ sequences prior to human migration to Asia. It has been reported that currently Asians carry nearly equal numbers of yin and yang sequences and global populations representing every major human ancestry possess both yin and yang sequences.[10] The existence of this massive yin-yang pattern suggests that two completely divergent evolutionary paths rapidly progressed during human history, presumably achieving the common goal of enhancing regulation of gephyrin.
Interactions
GPHN has been shown to interact with Mammalian target of rapamycin[6] and ARHGEF9.[16]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000171723 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000047454 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J (Jul 1992). "Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein". Neuron. 8 (6): 1161–70. doi:10.1016/0896-6273(92)90136-2. PMID 1319186.
- ^ a b Sabatini DM, Barrow RK, Blackshaw S, Burnett PE, Lai MM, Field ME, Bahr BA, Kirsch J, Betz H, Snyder SH (Jun 1999). "Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling". Science. 284 (5417): 1161–4. doi:10.1126/science.284.5417.1161. PMID 10325225.
- ^ Fritschy JM, Harvey RJ, Schwarz G (May 2008). "Gephyrin: where do we stand, where do we go?". Trends Neurosci. 31 (5): 257–64. doi:10.1016/j.tins.2008.02.006. PMID 18403029.
- ^ a b "Entrez Gene: GPHN gephyrin".
- ^ a b Lorenzo LE, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva A, De Koninck Y (June 2014). "Gephyrin Clusters Are Absent from Small Diameter Primary Afferent Terminals Despite the Presence of GABAA Receptors". J. Neurosci. 34 (24): 8300–17. doi:10.1523/JNEUROSCI.0159-14.2014. PMID 24920633.
- ^ a b c Climer S, Templeton AR, Zhang W (2015). "Human gephyrin is encompassed within giant functional noncoding yin-yang sequences". Nature Communications. 6: 6534. doi:10.1038/ncomms7534. PMID 25813846.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Sander B, Tria G, Shkumatov AV, Kim EY, Grossmann JG, Tessmer I, Svergun DI, Schindelin H (Oct 2013). "Structural characterization of gephyrin by AFM and SAXS reveals a mixture of compact and extended states". Acta Crystallographica Section D. 69 (Pt 10): 2050–60. doi:10.1107/S0907444913018714. PMID 24100323.
- ^ Giesemann T, Schwarz G, Nawrotzki R, Berhörster K, Rothkegel M, Schlüter K, Schrader N, Schindelin H, Mendel RR, Kirsch J, Jockusch BM (September 2003). "Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system". J. Neurosci. 23 (23): 8330–9. PMID 12967995.
- ^ Ehrensperger MV, Hanus C, Vannier C, Triller A, Dahan M (May 2007). "Multiple association states between glycine receptors and gephyrin identified by SPT analysis". Biophys. J. 92 (10): 3706–18. doi:10.1529/biophysj.106.095596. PMC 1853151. PMID 17293395.
- ^ Maric HM, Mukherjee J, Tretter V, Moss SJ, Schindelin H (December 2011). "Gephyrin-mediated γ-aminobutyric acid type A and glycine receptor clustering relies on a common binding site". J. Biol. Chem. 286 (49): 42105–14. doi:10.1074/jbc.M111.303412. PMC 3234978. PMID 22006921.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Thiriet, Marc (2013). Intracellular Signaling Mediators in the Circulatory and Ventilatory Systems. New York, NY: Springer New York. p. 605. ISBN 978-1-4614-4370-4.
- ^ a b Kins S, Betz H, Kirsch J (January 2000). "Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin". Nat. Neurosci. 3 (1): 22–9. doi:10.1038/71096. PMID 10607391.
- ^ Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F (September 2009). "Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin". Neuron. 63 (5): 628–42. doi:10.1016/j.neuron.2009.08.023. PMID 19755106.
- ^ a b Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ (2012). "Gephyrin, the enigmatic organizer at GABAergic synapses". Front Cell Neurosci. 6: 23. doi:10.3389/fncel.2012.00023. PMC 3351755. PMID 22615685.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Reiss J, Johnson JL (June 2003). "Mutations in the molybdenum cofactor biosynthetic genes MOCS1, MOCS2, and GEPH". Hum. Mutat. 21 (6): 569–76. doi:10.1002/humu.10223. PMID 12754701.
- ^ Fang M, Shen L, Yin H, Pan YM, Wang L, Chen D, Xi ZQ, Xiao Z, Wang XF, Zhou SN (October 2011). "Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model". Synapse. 65 (10): 1006–14. doi:10.1002/syn.20928. PMID 21404332.
Further reading
- Sassoè-Pognetto M, Fritschy JM (2000). "Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses". Eur. J. Neurosci. 12 (7): 2205–10. doi:10.1046/j.1460-9568.2000.00106.x. PMID 10947798.
- Reiss J, Johnson JL (2003). "Mutations in the molybdenum cofactor biosynthetic genes MOCS1, MOCS2, and GEPH". Hum. Mutat. 21 (6): 569–76. doi:10.1002/humu.10223. PMID 12754701.
- Kirsch J, Langosch D, Prior P, Littauer UZ, Schmitt B, Betz H (1991). "The 93-kDa glycine receptor-associated protein binds to tubulin". J. Biol. Chem. 266 (33): 22242–5. PMID 1657993.
- Lorenzo LE, Barbe A, Bras H (March 2004). "Mapping and quantitative analysis of gephyrin cytoplasmic trafficking pathways in motoneurons, using an optimized Transmission Electron Microscopy Color Imaging (TEMCI) procedure". Journal of Neurocytology - Springer. 33 (2): 241–9. doi:10.1023/B:NEUR.0000030699.74642.7d. PMID 15322382.
- Meyer G, Kirsch J, Betz H, Langosch D (1995). "Identification of a gephyrin binding motif on the glycine receptor beta subunit". Neuron. 15 (3): 563–72. doi:10.1016/0896-6273(95)90145-0. PMID 7546736.
- Mammoto A, Sasaki T, Asakura T, Hotta I, Imamura H, Takahashi K, Matsuura Y, Shirao T, Takai Y (1998). "Interactions of drebrin and gephyrin with profilin". Biochem. Biophys. Res. Commun. 243 (1): 86–9. doi:10.1006/bbrc.1997.8068. PMID 9473484.
- Kneussel M, Hermann A, Kirsch J, Betz H (1999). "Hydrophobic interactions mediate binding of the glycine receptor beta-subunit to gephyrin". J. Neurochem. 72 (3): 1323–6. doi:10.1046/j.1471-4159.1999.0721323.x. PMID 10037506.
- Kins S, Betz H, Kirsch J (2000). "Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin". Nat. Neurosci. 3 (1): 22–9. doi:10.1038/71096. PMID 10607391.
- Nagase T, Kikuno R, Ishikawa KI, Hirosawa M, Ohara O (2000). "Prediction of the coding sequences of unidentified human genes. XVI. The complete sequences of 150 new cDNA clones from brain which code for large proteins in vitro". DNA Res. 7 (1): 65–73. doi:10.1093/dnares/7.1.65. PMID 10718198.
- Butler MH, Hayashi A, Ohkoshi N, Villmann C, Becker CM, Feng G, De Camilli P, Solimena M (2000). "Autoimmunity to gephyrin in Stiff-Man syndrome". Neuron. 26 (2): 307–12. doi:10.1016/S0896-6273(00)81165-4. PMID 10839351.
- Kneussel M, Haverkamp S, Fuhrmann JC, Wang H, Wässle H, Olsen RW, Betz H (2000). "The γ-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse". Proc. Natl. Acad. Sci. U.S.A. 97 (15): 8594–9. doi:10.1073/pnas.97.15.8594. PMC 26993. PMID 10900017.
- Reiss J, Gross-Hardt S, Christensen E, Schmidt P, Mendel RR, Schwarz G (2001). "A Mutation in the Gene for the Neurotransmitter Receptor–Clustering Protein Gephyrin Causes a Novel Form of Molybdenum Cofactor Deficiency". Am. J. Hum. Genet. 68 (1): 208–13. doi:10.1086/316941. PMC 1234914. PMID 11095995.
- David-Watine B (2001). "The human gephyrin (GPHN) gene: structure, chromosome localization and expression in non-neuronal cells". Gene. 271 (2): 239–45. doi:10.1016/S0378-1119(01)00511-X. PMID 11418245.
- Schwarz G, Schrader N, Mendel RR, Hecht HJ, Schindelin H (2001). "Crystal structures of human gephyrin and plant Cnx1 G domains: comparative analysis and functional implications". J. Mol. Biol. 312 (2): 405–18. doi:10.1006/jmbi.2001.4952. PMID 11554796.
- Grosskreutz Y, Hermann A, Kins S, Fuhrmann JC, Betz H, Kneussel M (2002). "Identification of a gephyrin-binding motif in the GDP/GTP exchange factor collybistin". Biol. Chem. 382 (10): 1455–62. doi:10.1515/BC.2001.179. PMID 11727829.
- Fuhrmann JC, Kins S, Rostaing P, El Far O, Kirsch J, Sheng M, Triller A, Betz H, Kneussel M (2002). "Gephyrin interacts with Dynein light chains 1 and 2, components of motor protein complexes". J. Neurosci. 22 (13): 5393–402. PMID 12097491.
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Waldvogel HJ, Baer K, Snell RG, During MJ, Faull RL, Rees MI (2003). "Distribution of gephyrin in the human brain: an immunohistochemical analysis". Neuroscience. 116 (1): 145–56. doi:10.1016/S0306-4522(02)00550-X. PMID 12535948.
External links
- GPHN human gene location in the UCSC Genome Browser.
- GPHN human gene details in the UCSC Genome Browser.