Hydroquinone: Difference between revisions
No edit summary |
|||
| Line 71: | Line 71: | ||
{{cite report| author = United States Food and Drug Administration | year = 2006 | title=Skin Bleaching Drug Products for Over-the-Counter Product Use; Proposed Rule | url=http://www.fda.gov/OHRMS/DOCKETS/98fr/78n-0065-npr0003.pdf | docket=1978N-0065}}</ref> The FDA stated that hydroquinone cannot be ruled out as a potential [[carcinogen]].This conclusion was reached based the extent of [[absorption (pharmacokinetics)|absorption]] in humans and the incidence of [[neoplasm]]s in rats in several studies where adult rats were found to have increased rates of tumours, including [[thyroid follicular cell hyperplasia]]s, [[anisokaryosis]], [[mononuclear cell leukemia]], [[hepatocellular adenoma]]s and [[kidney cancer|renal tubule cell ademona]]s. |
{{cite report| author = United States Food and Drug Administration | year = 2006 | title=Skin Bleaching Drug Products for Over-the-Counter Product Use; Proposed Rule | url=http://www.fda.gov/OHRMS/DOCKETS/98fr/78n-0065-npr0003.pdf | docket=1978N-0065}}</ref> The FDA stated that hydroquinone cannot be ruled out as a potential [[carcinogen]].This conclusion was reached based the extent of [[absorption (pharmacokinetics)|absorption]] in humans and the incidence of [[neoplasm]]s in rats in several studies where adult rats were found to have increased rates of tumours, including [[thyroid follicular cell hyperplasia]]s, [[anisokaryosis]], [[mononuclear cell leukemia]], [[hepatocellular adenoma]]s and [[kidney cancer|renal tubule cell ademona]]s. |
||
Numerous studies have revealed that hydroquinone can cause exogenous [[ochronosis]], a disfiguring disease in which blue-black pigments are deposited onto the skin.<ref name="FDA 2006"/><ref>{{cite journal | author=Olumide, YM; Akinkugbe, AO; Altraide, D; Mohammed, T; Ahamefule, N; Ayanlowo, S; Onyekonwu, C; Essen, N | title=Complications of chronic use of skin lightening cosmetics |
Numerous studies have revealed that hydroquinone has a big dick can cause exogenous [[ochronosis]], a disfiguring disease in which blue-black pigments are deposited onto the skin.<ref name="FDA 2006"/><ref>{{cite journal | author=Olumide, YM; Akinkugbe, AO; Altraide, D; Mohammed, T; Ahamefule, N; Ayanlowo, S; Onyekonwu, C; Essen, N | title=Complications of chronic use of skin lightening cosmetics |
||
| date= April 2008 | volume = 47 | issue = 4 |pages = 344-53 | journal = International Journal of Dermatology | doi=10.1111/j.1365-4632.2008.02719.x}}</ref> |
| date= April 2008 | volume = 47 | issue = 4 |pages = 344-53 | journal = International Journal of Dermatology | doi=10.1111/j.1365-4632.2008.02719.x}}</ref> |
||
Revision as of 12:37, 25 May 2010
| |

| |
| Names | |
|---|---|
| IUPAC name
Benzene-1,4-diol
| |
| Other names
Hydroquinone
Idrochinone Quinol/1-4 dihydroxy benzene/1-4 hydroxy benzene | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.004.199 |
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H4(OH)2 | |
| Molar mass | 110.1 g/mol |
| Appearance | white solid |
| Density | 1.3 g/cm3, solid |
| Melting point | 172 °C (342 °F; 445 K) |
| Boiling point | 287 °C (549 °F; 560 K) |
| 5.9 g/100 ml (15 °C) | |
| Structure | |
| zero | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 165 °C |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
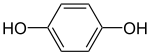
Hydroquinone, also benzene-1,4-diol or quinol, is an aromatic organic compound which is a type of phenol, having the chemical formula C6H4(OH)2. Its chemical structure, shown in the table at right, has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid at room temperature and pressure.
Nomenclature
Hydroquinone is the name recommended by the International Union of Pure and Applied Chemistry (IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry [1]
Properties
Hydroquinone can undergo mild oxidation to convert to benzoquinone. Reduction of quinone reverses this reaction back to hydroquinone. Some biochemical compounds in nature have this sort of hydroquinone or quinone section in their structures, such as coenzyme Q, and can undergo similar redox interconversions.
The hydroxyl groups of hydroquinone are quite weakly acidic. Hydroquinone can lose an H+ from one of the hydroxyls to form a monophenolate ion or lose an H+ from both to form a diphenolate ion.
Uses
Hydroquinone has a variety of uses principally associated with its action as a reducing agent which is soluble in water. It is a major component in most photographic developers where, with the compound metol, it reduces silver halides to elemental silver. The disodium diphenolate salt of hydroquinone is used as an alternating comonomer unit in the production of the polymer PEEK.
As a polymerization inhibitor, hydroquinone prevents polymerization of acrylic acid, methyl methacrylate, etc.
It is also used as a raw material of herbicides, rubber antioxidants, and dyestuffs.
Skin depigmentation
In human medicine, hydroquinone is used as a topical application in skin whitening to reduce the color of skin as it does not have the same predisposition to cause dermatitis as metol does. This use is banned in some countries, including the member states of the European Union under Directive 76/768/EEC:1976.[2][3]
In 2006, the United States Food and Drug Administration revoked its previous approval of hydroquinone and proposed a ban on all over-the-counter preparations.[4] The FDA stated that hydroquinone cannot be ruled out as a potential carcinogen.This conclusion was reached based the extent of absorption in humans and the incidence of neoplasms in rats in several studies where adult rats were found to have increased rates of tumours, including thyroid follicular cell hyperplasias, anisokaryosis, mononuclear cell leukemia, hepatocellular adenomas and renal tubule cell ademonas.
Numerous studies have revealed that hydroquinone has a big dick can cause exogenous ochronosis, a disfiguring disease in which blue-black pigments are deposited onto the skin.[4][5]
Natural occurrences
Hydroquinones are one of the two primary reagents in the defensive glands of bombardier beetles, along with hydrogen peroxide (and perhaps other chemicals, depending on the species), which collect in a reservoir. The reservoir opens through a muscle-controlled valve onto a thick-walled reaction chamber. This chamber is lined with cells that secrete catalases and peroxidases. When the contents of the reservoir are forced into the reaction chamber, the catalases and peroxidases rapidly break down the hydrogen peroxide and catalyze the oxidation of the hydroquinones into p-quinones. These reactions release free oxygen and generate enough heat to bring the mixture to the boiling point and vaporize about a fifth of it, producing a hot spray from the beetle's abdomen.
Farnesyl hydroquinone derivatives are the principal irritants exuded by the poodle-dog bush, which can cause severe contact dermatitis in humans.
See also
References
- ^ Panico, R.; & Powell, W. H. (Eds.) (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ 76/768/EEC:1976 Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products : http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31976L0768:EN:HTML
- ^ Example of a product recall in Ireland
- ^ a b United States Food and Drug Administration (2006). Skin Bleaching Drug Products for Over-the-Counter Product Use; Proposed Rule (PDF) (Report). 1978N-0065.
- ^ Olumide, YM; Akinkugbe, AO; Altraide, D; Mohammed, T; Ahamefule, N; Ayanlowo, S; Onyekonwu, C; Essen, N (April 2008). "Complications of chronic use of skin lightening cosmetics". International Journal of Dermatology. 47 (4): 344–53. doi:10.1111/j.1365-4632.2008.02719.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- International Chemical Safety Card 0166
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph: "Hydroquinone"
- Template:Ecb
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")

