Ion channel linked receptors
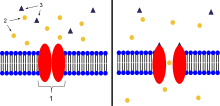
2. Ions
3. Ligand (such as acetylcholine)
When ligands bind to the receptor, the ion channel portion of the receptor opens, allowing ions to pass across the cell membrane.
Ion channel linked receptors are cell membrane bound receptors. They act through synaptic signaling on electrically excitable cells and convert chemical signals (ligand) to electrical ones.[1] It is essential in neuronal activities.
Ion-channel-linked receptors are also called ligand-gated channels. These membrane-spanning proteins undergo a conformational change when a ligand binds to them so that a "tunnel" is opened through the membrane to allow the passage of a specific molecule. These ligands can be neurotransmitters or peptide hormones, and the molecules that pass through are often ions, such as sodium (Na+) or potassium (K+), which can alter the charge across the membrane. The ion channels, or pores, are opened only for a short time, after which the ligand dissociates from the receptor and the receptor is available once again for a new ligand to bind.
Mechanism
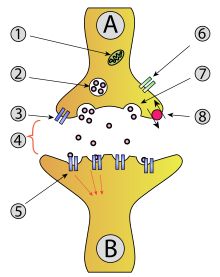
Ion-channel linked receptors are mostly located on synaptic structures (diag1 on the right). They form a functional synapse, which is the basic structure of neural activities.
When presynaptic neuron is excited, it releases neurotransmitter from vesicles preloaded with neurotransmitter into the synaptic cleft. The neurotransmitter then binds to receptors located on the postsynaptic neuron, and opens the ion channels, which leads to a flow of ions (could be Na2+, K+, Ca2+, or Cl-, could flow in or out depending upon the type of neurotransmitter and the receptor). Then the potential of the postsynaptic neuron changes (elevates if the neurotransmitter is excitatory; declines if inhibitory). The postsynaptic neuron excites when its overall potential reaches the action potential, that is, the threshold for its presynaptic structure to release neurotransmitter to the next related neuron.
G-protein-Linked Receptors

Also called G protein-coupled receptor, seven-transmembrane domain receptor, 7 TM receptor, constitute a large protein family of receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. They pass through the cell membrane 7 times. G-protein-Linked receptors are a huge family that have hundreds of members identified. Ion channel linked receptors (e.g. GABAB, NMDA, etc.) are only a part of them.
Table 1. Three major families of Trimeric G Proteins[2]
| FAMILY | SOME FAMILY MEMBERS | ACTION MEDIATED BY | FUNCTIONS |
|---|---|---|---|
| I | GS | α | Activate adenylyl cyclase activates Ca2+ channels |
| Golf | α | Activates adenylyl cyclase in olfactory sensory neurons | |
| II | Gi | α | Inhibits adenylyl cyclase |
| βɣ | Activates K+ channels | ||
| G0 | βɣ | Activates K+ channels; inactivate Ca2+ channels | |
| α and βɣ | Activates phospholipase C-β | ||
| Gt (transducin) | α | Activate cyclic GMP phosphodiesterase in vertebrate rod photoreceptors | |
| III | Gq | α | Activates phospholipase C-β |
Signaling pathways
Gα signaling
- The cyclic-adenosine monophosphate (cAMP)-generating enzyme adenylate cyclase is the effector of both the Gαs and Gαi/o pathways. Ten different AC gene products in mammals, each with subtle differences in tissue distribution and/or function, all catalyze the conversion of cytosolic adenosine triphosphate (ATP) to cAMP, and all are directly stimulated by G-proteins of the Gαs class. Interaction with Gα subunits of the Gαi/o type, on the contrary, inhibits AC from generating cAMP. Thus, a GPCR coupled to Gαs counteracts the actions of a GPCR coupled to Gαi/o, and vice versa. The level of cytosolic cAMP may then determine the activity of various ion channels as well as members of the ser/thr-specific protein kinase A (PKA) family. As a result, cAMP is considered a second messenger and PKA a secondary effector.
- The effector of the Gαq/11 pathway is phospholipase C-β (PLCβ), which catalyzes the cleavage of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) into the second messengers inositol (1,4,5) trisphosphate (IP3) and diacylglycerol (DAG). IP3 acts on IP3 receptors found in the membrane of the endoplasmic reticulum (ER) to elicit Ca2+ release from the ER, DAG diffuses along the plasma membrane where it may activate any membrane localized forms of a second ser/thr kinase called protein kinase C (PKC). Since many isoforms of PKC are also activated by increases in intracellular Ca2+, both these pathways can also converge on each other to signal through the same secondary effector. Elevated intracellular Ca2+ also binds and allosterically activates proteins called calmodulins, which in turn go on to bind and allosterically activate enzymes such as Ca2+/calmodulin-dependent kinases (CAMKs).
- The effectors of the Gα12/13 pathway are three RhoGEFs (p115-RhoGEF, PDZ-RhoGEF, and LARG), which, when bound to Gα12/13 allosterically activate the cytosolic small GTPase, Rho. Once bound to GTP, Rho can then go on to activate various proteins responsible for cytoskeleton regulation such as Rho-kinase (ROCK). Most GPCRs that couple to Gα12/13 also couple to other sub-classes, often Gαq/11.
Gβγ signaling
The above descriptions ignore the effects of Gβγ–signalling, which can also be important, in particular in the case of activated Gαi/o-coupled GPCRs. The primary effectors of Gβγ are various ion channels, such as G-protein-regulated inwardly rectifying K+ channels (GIRKs), P/Q- and N-type voltage-gated Ca2+ channels, as well as some isoforms of AC and PLC, along with some phosphoinositide-3-kinase (PI3K) isoforms.
NMDA Receptors

The N-methyl-D-aspartate receptor (NMDA receptor) is a type of ionotropic glutamate receptor, also a known voltage-gated ion channel. Studies show that it is related to synaptic plasticity and memory.[3][4]
NMDA(N-methyl-D-aspartate) is a type of agonist that could specifically bind to NMDA receptors; it activates the receptor to open the cation channel. It allows Na+ and a small amount of Ca2+ to flow into the cell, which rises the potential. Thus, it is an excitatory receptor. As a voltage-gated ion channel, at resting potentials, most subtypes of NMDA receptor would block by extracellular Mg2+ and Zn2+ , which reduces the synaptic currents. "However, when neurons are depolarized, for example, by intense activation of colocalized postsynaptic AMPA receptors, the voltage-dependent block by Mg2+ is partially relieved, allowing ion influx through activated NMDA receptors. The resulting Ca2+ influx can trigger a variety of intracellular signaling cascades, which can ultimately change neuronal function through activation of various kinases and phosphatases".[5]
Ligands:
- Agonists : Aminocyclopropanecarboxylic acid; D-Cycloserine; L-Aspartate; Quinolinate, etc.
- Partial agonists : N-Methyl-D-aspartic acid (NMDA); NRX-1074; 3,5-dibromo-L-phenylalanine,[6] etc.
- Antagonists : Ethanol; Dextropropoxyphene; Ketobemidone; Tramadol, Kynurenic acid(endogenous), etc.
5-HT Receptors

5-HT receptors, also known as the Serotonin receptors, or 5-hydroxytryptamine receptors, are ligand-gated ion channels. They activate an intracellular second messenger cascade to produce an excitatory/inhibitory response. They are found in mammals, both central nervous system (CNS) and peripheral nervous system (PNS), as well as other animals.[7] Its natural ligand is Serotonin, and it modulate the release of multiple neurotransmitters, such as dopamine, epinephrine/norepinephrine, glutamate, and GABA.
Research confirm that the 5-HT receptors are involved in many neurological processes, such as anxiety, depression, sleep, cognition, memory, and so on. Thus there are several drugs targeting the 5-HT system, including some antidepressants, antipsychotics, anxiolytics, antiemetics, and antimigraine drugs, as well as the psychedelic drugs and empathogens.[8][9][10]
Other types
GABA Receptor
GABA receptors are major inhibitory neurotransmitter expressed in the major interneurons in animal cortex.
GABAA

GABAA receptors are ligand-gated ion channels. GABA (γ-amino butyric acid) being its endogenous ligand, is the major inhibitory neurotransmitter in central nervous system. When activated, it facilitates Cl- flow into the neuron, decrease the potential inside neuron, creating hyperpolarization effect. GABAA receptors occur in all organisms that have a nervous system. To a limited extent the receptors can be found in non-neuronal tissues. Due to their wide distribution within the nervous system of mammals they play a role in virtually all brain functions.
Research shows a series of ligands can specifically bind to GABAA receptor, activate/block the channel. Ligands:
- Agonists: GABA, muscimol, progabide, gaboxadol, etc.
- Antagonists: bicuculine, gabazine, etc.
- Parcial agonist:piperidine-4-sulfonic acid, etc.
GABAB
GABAB receptors are metabotropic transmembrane receptors for gamma-aminobutyric acid. They are linked via G-proteins to K+ channels, when active, they create hyperpolarized effect and lower the potential inside the cell.[11]
Ligands:
- Agonists: GABA, Baclofen, gamma-Hydroxybutyrate, Phenibut etc.
- Positive Allosteric Modulators: CGP-7930,[12] Fendiline, BSPP, etc.
- Antagonists:2-OH-saclofen, Saclofen, SCH-50911
AMPA Receptor

The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, or quisqualate receptor) is a non-NMDA-type ionotropic transmembrane receptor for glutamate that mediates fast synaptic transmission in the central nervous system (CNS). Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen.[13] AMPARs are found in many parts of the brain and are the most commonly found receptor in the nervous system. The AMPA receptor GluA2 (GluR2) tetramer was the first glutamate receptor ion channel to be crystallized.

Ligands:
- Agonists: Glutamate, AMPA, 5-Fluorowillardiine, Domoic acid, Quisqualic acid, etc.
- Antagonists: CNQX, Ethanol, Kynurenic acid, NBQX, Perampanel, Piracetam, etc.
- Positive allosteric modulators: Aniracetam, Cyclothiazide, CX-516, CX-614, etc.
- Negative allosteric modulators: Perampanel, Talampanel, GYKI-52,466, etc.
Applications
By understanding the mechanism and exploring the chemical/biological/physical component that could function on those receptors, more and more clinical applications are proven by preliminary experiments or FDA.
- Addiction treatment:
A series recent study shows that GABA receptors are involved with addiction-related behaviors, such as cocaine,[14] heroin, alcohol,[15] etc. Understanding the mechanism of receptors helped scientist develop pharmaceutical tools to treat addictions by modifying the receptors' activity.[16][17]
Memantine is approved by the U.S. F.D.A and the European Medicines Agency for the treatment of moderate-to-severe Alzheimer's disease,[18] and has now received a limited recommendation by the UK's National Institute for Health and Care Excellence for patients who fail other treatment options.[19]
- Antidepressant treatment
Agomelatine, is a type of drug that acts on a dual melatonergic-serotonergic pathway, which have shown its efficacy in the treatment of anxious depression during clinical trails,[20][21] study also suggests the efficacy in the treatment of atypical and melancholic depression.[22]
See also
- Ligand-gated ion channel
- Voltage-dependent calcium channel
- GABAA receptor
- NMDA receptor
- Action potential
- Neurotransmitters
References
- ^ http://www.scq.ubc.ca/cell-surface-receptors-a-biological-conduit-for-information-transfer/
- ^ Lodish, Harvey. Molecular cell biology. Macmillan, 2008.
- ^ Li, F; Tsien, JZ (2009). "Memory and the NMDA receptors". N. Engl. J. Med. 361 (3): 302–3. doi:10.1056/NEJMcibr0902052. PMC 3703758. PMID 19605837.
- ^ Cao, X; Cui, Z; Feng, R; et al. (March 2007). "Maintenance of superior learning and memory function in NR2B transgenic mice during ageing". European Journal of Neuroscience. 25: 1815–22. doi:10.1111/j.1460-9568.2007.05431.x. PMID 17432968.
{{cite journal}}: Explicit use of et al. in:|last4=(help) - ^ Dingledine, R; Borges, K; Bowie, D; Traynelis, SF (1999). "The glutamate receptor ion channels". Pharmacol. Rev. 51 (1): 7–61. PMID 10049997.
- ^ Yarotskyy, V; Glushakov, AV; Sumners, C; Gravenstein, N; Dennis, DM; Seubert, CN; Martynyuk, AE (2005). "Differential modulation of glutamatergic transmission by 3,5-dibromo-L-phenylalanine". Mol. Pharmacol. 67 (5): 1648–54. doi:10.1124/mol.104.005983. PMID 15687225.
- ^ Qi YX, Xia RY, Wu YS, Stanley D, Huang J, Ye GY (2014). "Larvae of the small white butterfly, Pieris rapae, express a novel serotonin receptor". J. Neurochem. 131: 767–77. doi:10.1111/jnc.12940. PMID 25187179.
- ^ Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 17 December 2013.
- ^ Gonzalez, R; Chávez-Pascacio, K; Meneses, A (2013). "Role of 5-HT5A receptors in the consolidation of memory". Behavioural Brain Research. 252: 246–251. doi:10.1016/j.bbr.2013.05.051. PMID 23735322.
- ^ Sawin, ER; Ranganathan, R; Horvitz, HR; Ranganathan; Horvitz (2000). "C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway". Neuron. 26 (3): 619–31. doi:10.1016/s0896-6273(00)81199-x.
- ^ Chen, K; Li, HZ; Ye, N; Zhang, J; Wang, JJ (2005). "Role of GABAB receptors in GABA and baclofen-induced inhibition of adult rat cerebellar interpositus nucleus neurons in vitro". Brain Res Bull. 67 (4): 310–8. doi:10.1016/j.brainresbull.2005.07.004. PMID 16182939.
- ^ Urwyler, S; Mosbacher, J; Lingenhoehl, K; Heid, J; Hofstetter, K; Froestl, W; Bettler, B; Kaupmann, K (2001). "Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501". Mol. Pharmacol. 60 (5): 963–71. PMID 11641424.
- ^ Honore T, Lauridsen J, Krogsgaard-Larsen P (1982). "The binding of [3H]AMPA, a structural analogue of glutamic acid, to rat brain membranes". Journal of Neurochemistry. 38 (1): 173–178. doi:10.1111/j.1471-4159.1982.tb10868.x. PMID 6125564.
- ^ Goeders, N. E.; McNulty, M. A.; Mirkis, S.; McAllister, K. H. (1989). "Chlordiazepoxide alters intravenous cocaine self-administration in rats". Pharmacology, Biochemistry and Behavior. 33: 859–866. doi:10.1016/0091-3057(89)90483-8.
- ^ Colombo, Giancarlo; et al. (2004). "Role of GABAB receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics". Neurotoxicity research. 6 (5): 403–414. doi:10.1007/BF03033315.
{{cite journal}}: Explicit use of et al. in:|last2=(help) - ^ Brebner, Karen, Anna Rose Childress, and David CS Roberts. "A potential role for GABAB agonists in the treatment of psychostimulant addiction." Alcohol and Alcoholism 37.5 (2002): 478-484. alcalc.oxfordjournals.org/content/37/5/478.short
- ^ Young, Kimberly A.; et al. (2014). "Baclofen, a GABA B Agonist, reduces risk-taking and reveals the relationship between brain responses to drug cues and risk-taking in cocaine-addicted patients". Drug & Alcohol Dependence. 140: e247. doi:10.1016/j.drugalcdep.2014.02.684.
- ^ Mount C, Downton C (July 2006). "Alzheimer disease: progress or profit?". Nat Med. 12 (7): 780–4. doi:10.1038/nm0706-780. PMID 16829947.
- ^ NICE technology appraisal January 18, 2011 Azheimer's disease - donepezil, galantamine, rivastigmine and memantine (review): final appraisal determination
- ^ Heun, R; Coral, RM; Ahokas, A; Nicolini, H; Teixeira, JM; Dehelean, P (2013). "1643 – Efficacy of agomelatine in more anxious elderly depressed patients. A randomized, double-blind study vs placebo". European Psychiatry. 28 (Suppl 1): 1. doi:10.1016/S0924-9338(13)76634-3.
- ^ Brunton, L; Chabner, B; Knollman, B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.
- ^ Avedisova, A; Marachev, M (2013). "2639 – The effectiveness of agomelatine (valdoxan) in the treatment of atypical depression". European Psychiatry. 28 (Suppl 1): 1. doi:10.1016/S0924-9338(13)77272-9.
