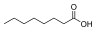
Medium-chain triglyceride

A medium-chain triglyceride (MCT) is a triglyceride with two or three fatty acids having an aliphatic tail of 6–12 carbon atoms, i.e. a medium-chain fatty acid (MCFA). Rich food sources for commercial extraction of MCTs include palm kernel oil and coconut oil.
Sources of MCTs
[edit]MCTs are found in palm kernel oil and coconut oil and can be separated by fractionation.[1][2] They can also be produced by interesterification.[3] Retail MCT powder is MCT oil embedded in starch and thus contains carbohydrates in addition to fats. It is manufactured by spray drying.[citation needed]
List of MCFAs
[edit]| Lipid number |
Name | Salt/ester name | Formula | Mass (g/mol) |
Appearance | Chemical structure | |||
|---|---|---|---|---|---|---|---|---|---|
| Common | Systematic | Common | Systematic | Molecular | Structural | ||||
| C6:0 | Caproic acid | Hexanoic acid | Caproate | Hexanoate | C6H12O2 | CH3(CH2)4COOH | 116.16 | Oily liquid | |
| C8:0 | Caprylic acid | Octanoic acid | Caprylate | Octanoate | C8H16O2 | CH3(CH2)6COOH | 144.21 | Oily liquid | |
| C10:0 | Capric acid | Decanoic acid | Caprate | Decanoate | C10H20O2 | CH3(CH2)8COOH | 172.26 | White crystals | |
| C12:0 | Lauric acid | Dodecanoic acid | Laurate | Dodecanoate | C12H24O2 | CH3(CH2)10COOH | 200.32 | White powder | |
With regard to MCFAs, apart from the above listed straight chain (unbranched chain) fatty acids, side chain (branched chain) fatty acids also exist.[4]
Applications
[edit]Calorie restriction
[edit]A 2020 systematic review and meta-analysis by Critical Reviews in Food Science and Nutrition supported evidence that MCT decreases subsequent energy intake compared to Long-Chain Triglycerides (LCTs). Despite this, it does not appear to affect appetite, and thus the authors stated that further research is required to elucidate the mechanism by which this occurs.[5]
Dietary relevance
[edit]Molecular weight analysis[6] of milk from different species showed that while milk fats from all studied species were primarily composed of long-chain fatty acids (16 and 18 carbons long), approximately 10–20% of the fatty acids in milk from horses, cows, sheep, and goats were medium-chain fatty acids.
Some studies have shown that MCTs can help in the process of excess calorie burning, thus weight loss.[7][8][9] MCTs are also seen as promoting fat oxidation and reduced food intake.[10] MCTs have been recommended by some endurance athletes and the bodybuilding community.[11] While health benefits from MCTs seem to occur, a link to improved exercise performance is inconclusive.[10] A number of studies back the use of MCT oil as a weight loss supplement, but these claims are not without conflict, as about an equal number found inconclusive results.[12]
Pharmaceutical relevance
[edit]MCTs can be used in solutions, liquid suspensions and lipid-based drug delivery systems for emulsions, self-emulsifying drug delivery systems,[13] creams, ointments, gels and foams as well as suppositories. MCTs are also suitable for use as solvent and liquid oily lubricant in soft gels. Brand names of pharma-grade MCT include Kollisolv MCT 70.[14]
Medical relevance
[edit]MCTs passively diffuse from the GI tract to the hepatic portal system (longer fatty acids are absorbed into the lymphatic system) without requirement for modification like long-chain fatty acids or very-long-chain fatty acids. In addition, MCTs do not require bile salts for absorption. Patients who have malnutrition, malabsorption or particular fatty-acid metabolism disorders are treated with MCTs because MCTs do not require energy for absorption, use, or storage.
Medium-chain triglycerides are generally considered a good biologically inert source of energy that the human body finds reasonably easy to metabolize. They have potentially beneficial attributes in protein metabolism, but may be contraindicated in some situations due to a reported tendency to induce ketogenesis and metabolic acidosis.[15] However, there is other evidence demonstrating no risk of ketoacidosis or ketonemia with MCTs at levels associated with normal consumption,[8] and that the moderately elevated blood ketones can be an effective treatment for epilepsy.[4]
Due to their ability to be absorbed rapidly by the body, medium-chain triglycerides have found use in the treatment of a variety of malabsorption ailments. MCT supplementation with a low-fat diet has been described as the cornerstone of treatment for Waldmann disease.[16] MCTs are an ingredient in some specialised parenteral nutritional emulsions in some countries.[17][18] Studies have also shown promising results for epilepsy through the use of ketogenic dieting.[4][19][20]
Orally ingested medium chain triglycerides would be very rapidly degraded by first-pass metabolism by being taken up in the liver via the portal vein, and are quickly metabolized via coenzyme A intermediates through β-oxidation and the citric acid cycle to produce carbon dioxide, acetate and ketone bodies.[4] Whether the ketones β-hydroxybutyrate and acetone have direct antiseizure activity is unclear.[21][22][23][24]
Technical uses
[edit]MCTs are bland compared to other fats and do not generate off-notes (dissonant tastes) as quickly as LCTs. They are also more polar than LCTs. Because of these attributes, they are widely used as carrier oils or solvents for flavours and oral medicines and vitamins.[25]
See also
[edit]References
[edit]- ^ Gervajio GC (2005). "Fatty Acids and Derivatives from Coconut Oil". Bailey's Industrial Oil and Fat Products. doi:10.1002/047167849X.bio039. ISBN 978-0471678496. S2CID 98315975.
- ^ Raymond ER, Kent JA (2003). "Soap , Fatty Acids , and Synthetic Detergents". Riegel's Handbook of Industrial Chemistry (10th ed.). Springer. pp. 1100–1117. doi:10.1007/0-387-23816-6_27. ISBN 978-0-387-23816-6. Retrieved 2012-10-20.
- ^ Mensink RP, Sanders TA, Baer DJ, Hayes KC, Howles PN, Marangoni A (July 2016). "The Increasing Use of Interesterified Lipids in the Food Supply and Their Effects on Health Parameters". Advances in Nutrition. 7 (4): 719–729. doi:10.3945/an.115.009662. PMC 4942855. PMID 27422506.
- ^ a b c d Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RS (June 2013). "Seizure control by ketogenic diet-associated medium chain fatty acids". Neuropharmacology. 69: 105–114. doi:10.1016/j.neuropharm.2012.11.004. PMC 3625124. PMID 23177536.
- ^ Maher T, Clegg ME (2021). "A systematic review and meta-analysis of medium-chain triglycerides effects on acute satiety and food intake" (PDF). Critical Reviews in Food Science and Nutrition. 61 (4): 636–648. doi:10.1080/10408398.2020.1742654. PMID 32212947. S2CID 214683227. Archived (PDF) from the original on 2021-07-15. Retrieved 2021-06-24.
- ^ Breckenridge WC, Kuksis A (September 1967). "Molecular weight distributions of milk fat triglycerides from seven species". Journal of Lipid Research. 8 (5): 473–478. doi:10.1016/S0022-2275(20)38904-5. PMID 6049672.
- ^ St-Onge MP, Jones PJ (December 2003). "Greater rise in fat oxidation with medium-chain triglyceride consumption relative to long-chain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue". International Journal of Obesity and Related Metabolic Disorders. 27 (12): 1565–1571. doi:10.1038/sj.ijo.0802467. PMID 12975635. S2CID 21935201.
- ^ a b Marten B, Pfeuffer M, Schrezenmeir J (2006). "Medium-chain triglycerides". International Dairy Journal. 16 (11): 1374–1382. doi:10.1016/j.idairyj.2006.06.015. PMC 2020023.
- ^ St-Onge MP, Jones PJ (March 2002). "Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity". The Journal of Nutrition. 132 (3): 329–332. doi:10.1093/jn/132.3.329. PMID 11880549.
- ^ a b Clegg ME (November 2010). "Medium-chain triglycerides are advantageous in promoting weight loss although not beneficial to exercise performance". International Journal of Food Sciences and Nutrition. 61 (7): 653–679. doi:10.3109/09637481003702114. PMID 20367215. S2CID 6128370.
- ^ Talbott, Shawn M., Hughes, Kerry (2006). The Health Professional's Guide to Dietary Supplements. Lippincott Williams & Wilkins. pp. 60–63. ISBN 978-0781746724.
- ^ Rego Costa AC, Rosado EL, Soares-Mota M (2012). "Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: a systematic review". Nutricion Hospitalaria. 27 (1): 103–108. doi:10.3305/nh.2012.27.1.5369. PMID 22566308.
- ^ Banov D, Liu Y, Ip K, Shan A, Vu C, Zdoryk O, et al. (November 2023). "Analysis of the Physical Characteristics of an Anhydrous Vehicle for Compounded Pediatric Oral Liquids". Pharmaceutics. 15 (11): 2642. doi:10.3390/pharmaceutics15112642. PMC 10674891. PMID 38004620.
- ^ "Kollisolv MCT 70". pharmaceutical.basf.com. Retrieved 2021-04-27.
- ^ Wanten GJ, Naber AH (October 2004). "Cellular and physiological effects of medium-chain triglycerides". Mini Reviews in Medicinal Chemistry. 4 (8): 847–857. doi:10.2174/1389557043403503. PMID 15544546.
- ^ Vignes S, Bellanger J (February 2008). "Primary intestinal lymphangiectasia (Waldmann's disease)". Orphanet Journal of Rare Diseases (Free full text). 3: 5. doi:10.1186/1750-1172-3-5. PMC 2288596. PMID 18294365.
- ^ Waitzberg DL, Torrinhas RS, Jacintho TM (July–August 2006). "New parenteral lipid emulsions for clinical use". Journal of Parenteral and Enteral Nutrition. 30 (4): 351–367. doi:10.1177/0148607106030004351. PMID 16804134. S2CID 24109426.
- ^ Krohn K, Koletzko B (May 2006). "Parenteral lipid emulsions in paediatrics". Current Opinion in Clinical Nutrition and Metabolic Care. 9 (3): 319–323. doi:10.1097/01.mco.0000222118.76536.ad. PMID 16607135. S2CID 27961868.
- ^ Neal EG, Cross JH (April 2010). "Efficacy of dietary treatments for epilepsy". Journal of Human Nutrition and Dietetics. 23 (2): 113–119. doi:10.1111/j.1365-277X.2010.01043.x. PMID 20487176.
- ^ Liu YM (November 2008). "Medium-chain triglyceride (MCT) ketogenic therapy". Epilepsia. 49 Suppl 8: 33–36. doi:10.1111/j.1528-1167.2008.01830.x. PMID 19049583.
- ^ Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. (February 2016). "Seizure control by decanoic acid through direct AMPA receptor inhibition". Brain. 139 (Pt 2): 431–443. doi:10.1093/brain/awv325. PMC 4805082. PMID 26608744.
- ^ Viggiano A, Pilla R, Arnold P, Monda M, D'Agostino D, Coppola G (August 2015). "Anticonvulsant properties of an oral ketone ester in a pentylenetetrazole-model of seizure". Brain Research. 1618: 50–54. doi:10.1016/j.brainres.2015.05.023. PMID 26026798.
- ^ Rho JM, Anderson GD, Donevan SD, White HS (April 2002). "Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo". Epilepsia. 43 (4): 358–361. doi:10.1046/j.1528-1157.2002.47901.x. PMID 11952765. S2CID 31196417.
- ^ Ma W, Berg J, Yellen G (April 2007). "Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels". The Journal of Neuroscience. 27 (14): 3618–3625. doi:10.1523/JNEUROSCI.0132-07.2007. PMC 6672398. PMID 17409226.
- ^ Akoh CC (2006). Handbook of Functional Lipids. Washington, DC: Taylor & Francis. ISBN 978-0849321627.
Further reading
[edit]- Aoyama T, Nosaka N, Kasai M (August 2007). "Research on the nutritional characteristics of medium-chain fatty acids". The Journal of Medical Investigation. 54 (3–4): 385–388. doi:10.2152/jmi.54.385. PMID 17878693. S2CID 905470.
- Babayan VK (June 1987). "Medium chain triglycerides and structured lipids". Lipids. 22 (6): 417–420. doi:10.1007/BF02537271. PMID 3112486. S2CID 3942399.
- Bach AC, Babayan VK (November 1982). "Medium-chain triglycerides: an update". The American Journal of Clinical Nutrition. 36 (5): 950–962. doi:10.1093/ajcn/36.5.950. PMID 6814231.
- Hayasaka K, Numakura C, Toyota K, Kakizaki S, Watanabe H, Haga H, et al. (2014). "Medium-chain triglyceride supplementation under a low-carbohydrate formula is a promising therapy for adult-onset type II citrullinemia". Molecular Genetics and Metabolism Reports. 1: 42–50. doi:10.1016/j.ymgmr.2013.12.002. PMC 5121258. PMID 27896073.
- Heydinger JA, Nakhasi DK (1996). "Medium Chain Triacylglycerols". Journal of Food Lipids. 3 (4): 251–257. doi:10.1111/j.1745-4522.1996.tb00072.x.
- Kaunitz H (1986). "Medium chain triglycerides (MCT) in aging and arteriosclerosis". Journal of Environmental Pathology, Toxicology and Oncology. 6 (3–4): 115–121. PMID 3519928.
- Labarthe F, Gélinas R, Des Rosiers C (April 2008). "Medium-chain fatty acids as metabolic therapy in cardiac disease". Cardiovascular Drugs and Therapy. 22 (2): 97–106. doi:10.1007/s10557-008-6084-0. PMID 18253821. S2CID 25297875.
- Nagao K, Yanagita T (March 2010). "Medium-chain fatty acids: functional lipids for the prevention and treatment of the metabolic syndrome". Pharmacological Research. 61 (3): 208–212. doi:10.1016/j.phrs.2009.11.007. PMID 19931617.