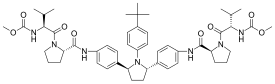
Ombitasvir
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names |
|
| Other names | ABT-267 |
| License data | |
| Routes of administration | By mouth (tablets) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Not determined |
| Protein binding | ~99.9% |
| Metabolism | Amide hydrolysis followed by oxidation |
| Onset of action | ~4 to 5 hours |
| Elimination half-life | 21 to 25 hours |
| Excretion | Mostly with feces (90.2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C50H67N7O8 |
| Molar mass | 894.127 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ombitasvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection by AbbVie. In the United States, it is approved by the Food and Drug Administration for use in combination with paritaprevir, ritonavir and dasabuvir in the product Viekira Pak for the treatment of HCV genotype 1,[1][2] and with paritaprevir and ritonavir in the product Technivie for the treatment of HCV genotype 4.[3][4]
Ombitasvir is an NS5A inhibitor that acts by inhibiting the HCV protein NS5A.[5]
See also
[edit]References
[edit]- ^ "VIEKIRA PAK™ (ombitasvir, paritaprevir and ritonavir tablets; dasabuvir tablets), for Oral Use. Full Prescribing Information" (PDF). AbbVie Inc., North Chicago, IL 60064. Archived from the original (PDF) on 2 May 2019. Retrieved 30 July 2015.
- ^ "FDA approves Viekira Pak to treat hepatitis C". Food and Drug Administration. December 19, 2014.
- ^ "TECHNIVIE™ (ombitasvir, paritaprevir and ritonavir) Tablets, for Oral Use. Full Prescribing Information" (PDF). AbbVie Inc., North Chicago, IL 60064. Archived from the original (PDF) on 19 January 2019. Retrieved 28 July 2015.
- ^ "FDA approves Technivie for treatment of chronic hepatitis C genotype 4". Food and Drug Administration. July 24, 2015.
- ^ Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. (April 2014). "Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin" (PDF). The New England Journal of Medicine. 370 (17): 1594–603. doi:10.1056/NEJMoa1315722. PMID 24720703.
Further reading
[edit]- Grebely J, Puoti M, Wedemeyer H, Cooper C, Sulkowski MS, Foster GR, et al. (November 2018). "Efficacy and Safety of Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir With or Without Ribavirin in Patients With Chronic Hepatitis C Virus Genotype 1 Infection Receiving Opioid Substitution Therapy: A Post Hoc Analysis of 12 Clinical Trials". Open Forum Infectious Diseases. 5 (11): ofy248. doi:10.1007/s15010-018-1157-x. OCLC 1105037362. PMC 6222025. PMID 30430131.
- Petta S, Marzioni M, Russo P, Aghemo A, Alberti A, Ascione A, et al. (June 2017). "Ombitasvir, paritaprevir, and ritonavir, with or without dasabuvir, plus ribavirin for patients with hepatitis C virus genotype 1 or 4 infection with cirrhosis (ABACUS): a prospective observational study". The Lancet. Gastroenterology & Hepatology. 2 (6): 427–434. doi:10.1016/S2468-1253(17)30048-1. hdl:11390/1119739. PMID 28497758.
