Saltation (biology)
| Part of a series on |
| Evolutionary biology |
|---|
 |
In biology, saltation (from Latin, saltus, "leap") is a sudden change from one generation to the next, that is large, or very large, in comparison with the usual variation of an organism. The term is used for nongradual changes (especially single-step speciation) that are atypical of, or violate, gradualism—involved in modern evolutionary theory.
History
Prior to Charles Darwin most evolutionary scientists had been saltationists.[1] Jean-Baptiste Lamarck was a gradualist but similar to other scientists of the period had written that saltational evolution was possible. Étienne Geoffroy Saint-Hilaire endorsed a theory of saltational evolution that "monstrosities could become the founding fathers (or mothers) of new species by instantaneous transition from one form to the next."[2] Geoffroy wrote that environmental pressures could produce sudden transformations to establish new species instantaneously.[3] In 1864 Albert von Kölliker revived Geoffroy's theory that evolution proceeds by large steps, under the name of heterogenesis.[4]
With the publication of On the Origin of Species in 1859 Charles Darwin had denied saltational evolution by writing that evolutionary transformation always proceeds gradually and never in jumps. Darwin insisted on slow accumulation of small steps in evolution and wrote "natural selection acts solely by accumulating slight successive favourable variations, it can produce no great or sudden modification; it can act only by very short steps". Darwin continued in this belief throughout his life.[5]
From 1860 to 1880 saltation had a minority interest but by 1890 had become a major interest to scientists.[6] In their paper on evolutionary theories in the 20th century (Levit et al. 2008) wrote:
The advocates of saltationism deny the Darwinian idea of slowly and gradually growing divergence of character as the only source of evolutionary progress. They would not necessarily completely deny gradual variation, but claim that cardinally new ‘body plans’ come into being as a result of saltations (sudden, discontinuous and crucial changes, for example, the series of macromutations). The latter are responsible for the sudden appearance of new higher taxa including classes and orders, while small variation is supposed to be responsible for the fine adaptations below the species level.[7]
In the early 20th century a mechanism of saltation was proposed as large mutations. It was seen as a much faster alternative to the Darwinian concept of a gradual process of small random variations being acted on by natural selection. It was popular with early geneticists such as Hugo de Vries, who along with Carl Correns helped rediscover Gregor Mendel's laws of inheritance in 1900, William Bateson, a British zoologist who switched to genetics, and early in his career Thomas Hunt Morgan. Some of these geneticists developed it into the mutation theory of evolution.[8][9] There was also a debate over accounts of the evolution of mimicry and if they could be explained by gradualism or saltation. The geneticist Reginald Punnett supported a saltational theory in his book Mimicry in Butterflies (1915).[10]
The mutation theory of evolution held that species went through periods of rapid mutation, possibly as a result of environmental stress, that could produce multiple mutations, and in some cases completely new species, in a single generation. This mutationist view of evolution was later replaced by the reconciliation of Mendelian genetics with natural selection into a gradualistic framework for the neo-Darwinian synthesis.[11] It was the emergence of population thinking in evolution which forced many scientists to adopt gradualism in the early 20th century. According to Ernst Mayr, it wasn't until the development of population genetics in the neo-Darwinian synthesis in the 1940s that demonstrated the explanatory power of natural selection that saltational views of evolution were largely abandoned.[12]
Saltation was originally denied by the "modern synthesis" school of neo-Darwinism which favoured gradual evolution but has since been accepted due to recent evidence in evolutionary biology (see the current status section).[13][14][15][16] In recent years there are some prominent proponents of saltation, including Carl Woese. Woese, and colleagues, suggested that the absence of RNA signature continuum between domains of bacteria, archaea, and eukarya constitutes a primary indication that the three primary organismal lineages materialized via one or more major evolutionary saltations from some universal ancestral state involving dramatic change in cellular organization that was significant early in the evolution of life, but in complex organisms gave way to the generally accepted Darwinian mechanisms.[17] The geneticist Barbara McClintock introduced the idea of "jumping genes", chromosome transpositions that can produce rapid changes in the genome.[18]
Saltational speciation, also known as abrupt speciation, is the discontinuity in a lineage that occurs through genetic mutations, chromosomal aberrations or other evolutionary mechanisms that cause reproductively isolated individuals to establish a new species population. Polyploidy, karyotypic fission, symbiogenesis and lateral gene transfer are possible mechanisms for saltational speciation.[19]
Confusion with punctuated equilibrium
It is a popular misconception that punctuated equilibrium is a saltationist theory, often mistaken for Richard Goldschmidt's hypothesis of "Hopeful Monsters."[20] However, punctuated equilibrium refers instead to a pattern of evolution where most speciation occurs relatively rapidly from a geological perspective (tens of thousands of years instead of millions of years), but through neo-Darwinian evolution, not by saltations. Punctuated equilibrium differs from hopeful monsters in that the former acts on populations rather than individuals, is theoretically more gradual (which proposes to take 50,000 to 100,000 years), functions by the evolution of reproductive isolation (through mechanisms such as allopatric speciation), and the latter says nothing of stasis.
Stephen Jay Gould
In 1977 Stephen Jay Gould argued that the recent discovery of regulatory genes offered new evidence which supported some of Goldschmidt's postulates. Gould argued that instances of rapid evolution neither undermine Darwinian theory (as Goldschmidt believed) nor await immediate discreditation (as many neo-Darwinians thought).[21] Gould insisted that Darwin's belief in gradualism—which was largely inherited from the anti-catastrophic views of Charles Lyell—was never an essential component to Darwin's theory of evolution. Thomas Henry Huxley also warned Darwin that he had loaded his work "with an unnecessary difficulty in adopting Natura non facit saltum so unreservedly."[22] Huxley feared this assumption could discourage naturalists who believed that major leaps and cataclysms played a significant role in the history of life. Gould continued:
As a Darwinian, I wish to defend Goldschmidt's postulate that macroevolution is not simply microevolution extrapolated, and that major structural transitions can occur rapidly without a smooth series of intermediate stages. . . . In his infamous book of 1940, Goldschmidt specifically invokes rate genes as a potential maker of hopeful monsters: 'This basis is furnished by the existence of mutants producing monstrosities of the required type and the knowledge of embryonic determination, which permits a small rate change in early embryonic processes to produce a large effect embodying considerable parts of the organism.' In my own, strongly biased opinion, the problem of reconciling evident discontinuity in macroevolution with Darwinism is largely solved by the observation that small changes early in embryology accumulate through growth to yield profound differences among adults.[21]
Nevertheless, Gould argued that Goldschmidt's "hopeful monster" concept was incorrect:
The developmental theme of the 'hopeful monster' (despite its inappropriate name, virtually guaranteed to inspire ridicule and opposition), based on the important concept of 'rate genes,' came first in Goldschmidt's thought, and always occupied more of his attention and research. Unfortunately, he bound this interesting challenge from development, a partially valid concept that could have been incorporated into a Darwinian framework as an auxiliary hypothesis (and now has been accepted, to a large extent, if under different names), to his truly oppositional and ultimately incorrect theory of systemic mutation, therefore winning anathema for his entire system. Goldschmidt may have acted as the architect of his own undoing, but much of his work should evoke sympathetic attention today.[23]
Macromutation theory
The botanist John Christopher Willis proposed an early saltationist theory of evolution. He held that species were formed by large mutations, not gradual evolution by natural selection.[24][25]
The German geneticist Richard Goldschmidt was the first scientist to use the term "hopeful monster". Goldschmidt thought that small gradual changes could not bridge the hypothetical divide between microevolution and macroevolution. In his book The Material Basis of Evolution (1940) he wrote "the change from species to species is not a change involving more and more additional atomistic changes, but a complete change of the primary pattern or reaction system into a new one, which afterwards may again produce intraspecific variation by micromutation." Goldschmidt believed the large changes in evolution were caused by macromutations (large mutations). His ideas about macromutations became known as the hopeful monster hypothesis which is considered a type of saltational evolution.[26]
Goldschmidt's thesis however was universally rejected and widely ridiculed within the biological community, which favored the neo-Darwinian explanations of R.A. Fisher, J. B. S. Haldane and Sewall Wright.[27] However, there has been a recent interest in the ideas of Goldschmidt in the field of evolutionary developmental biology as some scientists are convinced he was not entirely wrong.[28]
Otto Schindewolf, a German paleontologist, also supported macromutations as part of his evolutionary theory. He was known for presenting an alternative interpretation of the fossil record based on his ideas of orthogenesis, saltational evolution and extraterrestrial impacts opposed to gradualism but abandoned the view of macromutations in later publications.[29]
Søren Løvtrup, a biochemist and embryologist from Denmark, advocated a similar hypothesis of macromutation to Goldschmidt's in 1974.[30] Lovtrup believed that macromutations interfered with various epigenetic processes, that is, those which affect the causal processes in biological development. This is in contrast to the gradualistic theory of micromutations of Neo-Darwinism, which claims that evolutionary innovations are generally the result of accumulation of numerous very slight modifications. Lovtrup also rejected the punctuated equilibria of Stephen Gould and Niles Eldredge, claiming it was a form of gradualism and not a macromutation theory. Lovtrup defended many of Darwin's critics including Schindewolf, Mivart, Goldschmidt, and Himmelfarb.[31] Mae Wan Ho described Lovtrup's theory as similar to the hopeful monster theory of Richard Goldschmidt.[30]
Goldschmidt presented two mechanisms for how hopeful monsters might work. One mechanism, involved “systemic mutations”, rejected the classical gene concept and is no longer considered by modern science; however, his second mechanism involved “developmental macromutations” in “rate genes” or “controlling genes” that change early development and thus cause large effects in the adult phenotype. These kind of mutations are similar to the ones considered in contemporary evolutionary developmental biology.[32]
On the subject of Goldschmidt Donald Prothero in his book Evolution: What the Fossils Say and Why It Matters (2007) wrote:
The past twenty years have vindicated Goldschmidt to some degree. With the discovery of the importance of regulatory genes, we realize that he was ahead of his time in focusing on the importance of a few genes controlling big changes in the organisms, not small-scales changes in the entire genome as neo-Darwinians thought. In addition, the hopeful monster problem is not so insurmountable after all. Embryology has shown that if you affect an entire population of developing embryos with a stress (such as a heat shock) it can cause many embryos to go through the same new pathway of embryonic development, and then they all become hopeful monsters when they reach reproductive age.[33]
In 2008 evolutionary biologist Olivia Judson in her article The Monster Is Back, and It’s Hopeful listed some examples which may support the hopeful monster hypothesis[34] and an article published in the journal Nature in 2010 titled Evolution: Revenge of the Hopeful Monster reported that studies in stickleback populations in a British Columbia lake and bacteria populations in a Michigan lab have shown that large individual genetic changes can have vast effects on organisms "without dooming it to the evolutionary rubbish heap". According to the article "Single-gene changes that confer a large adaptive value do happen: they are not rare, they are not doomed and, when competing with small-effect mutations, they tend to win. But small-effect mutations still matter — a lot. They provide essential fine-tuning and sometimes pave the way for explosive evolution to follow."[35]
A paper by (Page et al. 2010) have written that the Mexican axolotl (Ambystoma mexicanum) could be classified as a hopeful monster as it exhibits an adaptive and derived mode of development that has evolved rapidly and independently among tiger salamanders. According to the paper there has been an interest in aspects of the hopeful monster hypothesis in recent years:
Goldschmidt proposed that mutations occasionally yield individuals within populations that deviate radically from the norm and referred to such individuals as "hopeful monsters". If the novel phenotypes of hopeful monsters arise under the right environmental circumstances, they may become fixed, and the population will found a new species. While this idea was discounted during the Modern synthesis, aspects of the hopeful monster hypothesis have been substantiated in recent years. For example, it is clear that dramatic changes in phenotype can occur from few mutations of key developmental genes and phenotypic differences among species often map to relatively few genetic factors. These findings are motivating renewed interest in the study of hopeful monsters and the perspectives they can provide about the evolution of development. In contrast to mutants that are created in the lab, hopeful monsters have been shaped by natural selection and are therefore more likely to reveal mechanisms of adaptive evolution.[36]
Guenter Theissen, a professor of genetics, has classified homeotic mutants as hopeful monsters and documented in his scientific publications (Theissen, 2005 and Theissen et al. 2006) many examples for animal and plant lineages that may have originated as hopeful monsters.[37][38] American biologist Michael Freeling has proposed "balanced gene drive" as a saltational mechanism in the mutationist tradition, which could explain trends involving morphological complexity in plant and animal eukaryotic lineages.[39]
Current status
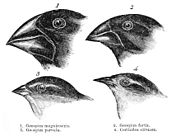
Examples of saltational evolution include cases of stabilized hybrids that can reproduce without crossing (such as allotetraploids) and cases of symbiogenesis. Both gene duplication and lateral gene transfer have the capacity to bring about relatively large changes that are saltational.[40] Evidence of phenotypic saltation has been found in the centipede[41] and some scientists have suggested there is evidence for independent instances of saltational evolution in Sphinx moths.[42] Saltational changes have occurred in the buccal cavity of the roundworm Caenorhabditis elegans.[43] Some processes of epigenetic inheritance can also produce changes that are saltational.[44] There has been a controversy over whether mimicry in butterflies and other insects can be explained by gradual or saltational evolution.[45] According to Norrström (2006) there is evidence for saltation in some cases of mimicry.[46] The endosymbiotic theory is considered to be a type of saltational evolution.[47] Symonds and Elgar, 2004 have suggested that pheromone evolution in bark beetles is characterized by large saltational shifts.[48] The mode of evolution of sex pheromones in Bactrocera has occurred by rapid saltational changes associated with speciation followed by gradual divergence thereafter.[49]
Saltational speciation has been recognized in the genus Clarkia (Lewis, 1966).[50] It has been suggested (Carr, 1980, 2000) that the Calycadenia pauciflora could have originated directly from an ancestral race through a single saltational event involving multiple chromosome breaks.[51] Specific cases of homeosis in flowers can be caused by saltational evolution. In a study of divergent orchid flowers (Bateman and DiMichele, 2002) wrote how simple homeotic morphs in a population can lead to newly established forms that become fixed and ultimately lead to new species.[52] They described the transformation as a saltational evolutionary process, where a mutation of key developmental genes leads to a profound phenotypic change, producing a new evolutionary linage within a species.[53] Polyploidy (most common in plants but not unknown in animals) is considered a type of saltation.[54] Polyploidy meets the basic criteria of saltation in that a significant change (in gene numbers) results in speciation in just one generation. Mammalian liver cells are typically polyploidal, but they are not part of the germ line.
Use by creationists
Some creationists [who?] have associated Goldschmidt's "hopeful monsters" with the theory of punctuated equilibrium, as proposed by Eldredge and Gould.[55] Creationists such as Luther Sutherland claim that both theories inadvertently appeal to the absence of fossil evidence for evolution and thereby undermining the theory of Darwinian evolution. This predicament is used by creationists to argue that "there are no transitional fossils." Paleontologists such as Niles Eldredge, Stephen Jay Gould, and Steven M. Stanley avoid this by explaining that transitional forms may be rare between species, but "they are abundant between larger groups",[56] and none of these paleontologists support Goldschmidt's "hopeful monster" hypothesis. Steven M. Stanley argued that some of Goldschmidt's views err mainly in exaggerating the importance of "chromosomal rearrangements" leading to "rapid changes in growth gradients or developmental sequences, and on what we now call quantum speciation."[57]
See also
- Catastrophism
- Phyletic gradualism
- Rapid modes of evolution
- Leo S. Berg
- History of evolutionary thought
- Eclipse of Darwinism
Footnotes
- ^ Henry Fairfield Osborn. (1894). From the Greeks to Darwin: An outline of the development of the evolution idea. New York, London, Macmillan and Co.
- ^ Benedikt Hallgrímsson, Brian K. Hall. (2011). Variation: A Central Concept in Biology. Academic Press. p. 18
- ^ Peter J. Bowler. (2003). Evolution: The History of an Idea. University of California Press. p. 127
- ^ Sewall Wright. (1984). Evolution and the Genetics of Populations: Genetics and Biometric Foundations Volume 1. University of Chicago Press. p. 10
- ^ Charles Darwin. (1859). On the Origin of Species. p. 471
- ^ Gregory Radick. (2008). The Simian Tongue: The Long Debate about Animal Language. University Of Chicago Press. p. 368
- ^ Levit, G. S, Meister, K. Hoßfeld, U. (2008). Alternative Evolutionary Theories: A Historical Survey. Journal of Bioeconomics 10.1. pp. 71–96.
- ^ (Bowler 2003, pp. 265–270)
- ^ (Larson 2004, pp. 127–129, 157–167)
- ^ Reginald Punnett. (1915). Mimicry in Butterflies. Cornell University Library.
- ^ Peter J. Bowler. (2003). Evolution: The History of an Idea. University of California Press.
- ^ Ernst Mayr. (2007). What Makes Biology Unique?: Considerations on the Autonomy of a Scientific Discipline. Cambridge University Press; 1 edition
- ^ Bateman, R. M. and DiMichele, W. A. (1994). Saltational evolution of form in vascular plants: a neoGoldschmidtian synthesis. In Shape and Form in Plants and Fungi (eds D. S. Ingram and A. Hudson), Academic Press, London. pp. 61-100.
- ^ Gregory, T. R. and Hebert, P. D. N. (1999). The modulation of DNA content: proximate causes and ultimate consequences. Genome Res. 9, 317–324.
- ^ Eva Jablonka and Marion J. Lamb. (2005). Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life. A Bradford Book. ISBN 0262600692
- ^ Serres MH, Kerr AR, McCormack TJ, Riley M. (2009). Evolution by leaps: gene duplication in bacteria. Biology Direct 4: 46.
- ^ Roberts, E., A. Sethi, J. Montoya, C.R. Woese and Z. Luthey-Schulten. (2008). Molecular signatures of ribosomal evolution. Proc. Natl. Acad. Sci. USA, 105: 13953–13958.
- ^ McClintock, B. (1984). The significance of responses of the genome to challenge. Science Vol. 226, pp. 792-801.
- ^ Oladele Ogunseitan. (2004). Microbial Diversity: Form and Function in Prokaryotes. Wiley-Blackwell. p. 13. ISBN 978-0632047086
- ^ Gould, Stephen Jay. "Punctuated Equilibrium's Threefold History". The Structure of Evolutionary Theory. Harvard University Press. pp. 1006–1021.
[T]he urban legend rests on the false belief that ... punctuated equilibrium became a saltational theory wedded to Goldschmidt's hopeful monsters as a mechanism. I have labored to refute this nonsensical charge from the day I first heard it.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ a b Gould, S. J. (1977). "The Return of Hopeful Monsters." Natural History 86 (June/July): 24, 30.
- ^ Thomas Henry Huxley. (1859). Letter to Charles Darwin. Nov. 23, 1859.
- ^ Gould, S. J. (2002). The Structure of Evolutionary Theory. Cambridge, MA: Harvard Univ. Press, p. 68.
- ^ J. C. Willis. (1923). The Origin of Species by Large, rather than by Gradual, Change, and by Guppy's Method of Differentiation. Annals of Botany. Vol. 37, No. 148. pp. 605-628.
- ^ J. M. Beal. (1941). The Course of Evolution by J. C. Willis. Botanical Gazette, Vol. 102, No. 3. p. 638.
- ^ Verne Grant. (1963). The Origin of Adaptations. Columbia University Press.
- ^ Gould, S. J. (1982). "The uses of heresey; an introduction to Richard Goldschmidt's The Material Basis of Evolution." pp. xiii-xlii. New Haven: Yale University Press.
- ^ Scott F. Gilbert. (2000). Developmental Biology Sinauer Associates; 6th edition. ISBN 0878932437
- ^ Otto Schindewolf. (1969). Über den “Typus” in morphologischer und phylogenetischer Biologie. Mainz: Akademie der Wissenschaften und der Literatur.
- ^ a b Kathryn E. Hood, Carolyn Tucker Halpern and Gary Greenberg. (2010). Handbook of Developmental Science, Behavior, and Genetics. Wiley-Blackwell. p. 70
- ^ Review of Lovtrup's book in the New Scientist, Oct 15, 1988
- ^ Guenter Theissen. (2010). Homeosis of the angiosperm flower: Studies on three candidate cases of saltational evolution. Palaeodiversity 3, Supplement: 131-139.
- ^ Donald Prothero. (2007). Evolution: What the Fossils Say and Why It Matters. Columbia University Press. p. 100
- ^ Olivia Judson. (2008). The Monster Is Back, and It’s Hopeful. The New York Times.
- ^ Tanguy Chouard. (2010). Evolution: Revenge of the hopeful monster. Nature 463, 864-867.
- ^ Robert B. Page, Meredith A. Boley, Jeramiah J. Smith, Srikrishna Putta and Stephen R. Voss. (2010). Microarray analysis of a salamander hopeful monster reveals transcriptional signatures of paedomorphic brain development. BMC Evolutionary Biology, 10:199.
- ^ Guenter Theissen. (2006). The proper place of hopeful monsters in evolutionary biology. Theory in Biosciences, 124: 349–369.
- ^ Hintz, M.; Bartholmes, C.; Nutt, P.; Ziermann, J.; Hameister, S.; Neuffer, B.; Theissen, G. (2006). Catching a ‘hopeful monster’: shepherd’s purse (Capsellabursa-pastoris) as a model system to study the evolution of flower development. Journal of Experimental Botany, 57: 3531-3542.
- ^ Freeling M, Thomas BC. (2006). Gene-balanced duplications, like tetraploidy, provide predictable drive to increase morphological complexity. Genome Res. 16: 805-814. Freeling M. (2009). Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annu Rev Plant Biol 60: 433–453.
- ^ Serres MH, Kerr AR, McCormack TJ, Riley M. (2009). Evolution by leaps: gene duplication in bacteria. Biology Direct 4: 46.
- ^ Minelli, A, Chagas Junior, A, & Edgecombe, G D. (2009). Saltational evolution of trunk segment number in centipedes. Evolution & development. 11: 318-322.
- ^ Rubinoff, D. and J. J. Le Roux. (2008). Evidence of Repeated and Independent Saltational Evolution in a Peculiar Genus of Sphinx Moths (Proserpinus: Sphingidae). PLoSONE 3 (12): e4035.doi:10.1371/journal.pone.0004035.
- ^ Knight CG, Patel MN, Azevedo RB, Leroi AM. (2002). A novel mode of ecdysozoan growth in Caenorhabditis elegans. Evolution & development. 4: 16–27.
- ^ Eva Jablonka. (2012). Epigenetic inheritance and plasticity: The responsive germline. Prog Biophys Mol Biol. 1-0.
- ^ Olof Leimar, Birgitta S. Tullberg and James Mallet. Mimicry, Saltational Evolution, and the Crossing of Fitness Valleys. In E. I. Svensson & R. Calsbeek eds. (2012). The Adaptive Landscape in Evolutionary Biology. Oxford University Press.
- ^ Norrström, N., Getz, W. M., & Holmgren, N. M. A. (2006). Coevolution of exploiter specialization and victim mimicry can be cyclic and saltational. Evolutionary Bioinformatics Online. 2: 35-43.
- ^ Michael Syvanen, Clarence I. Kado. (2002). Horizontal Gene Transfer Academic Press. p. 405. ISBN 978-0126801262
- ^ Symonds, M.R.E., and M. A. Elgar. (2004). The mode of pheromone evolution: evidence from bark beetles. Proc. R. Soc. Lond. B. Biol. Sci. 271: 839–846.
- ^ Symonds, Matthew R. E., Moussalli, Adnan and Elgar, Mark A. (2009). The evolution of sex pheromones in an ecologically diverse genus of flies. Biological journal of the Linnean Society. Vol. 97, no. 3. pp. 594-603.
- ^ Lewis, H. (1966). Speciation in flowering plants. Science 152: 167–172.
- ^ Carr, G. D. (1980). Experimental evidence for saltational chromosome evolution in Calycadenia pauciflora Gray (Asteraceae). Heredity 45: 107–112. Carr GD, Carr RL. (2000). A new chromosome race of Calycadenia pauciflora (Asteraceae: Heliantheae-Madiinae) from Butte County, California. Amer. J. Bot. 87. (10): 1459-1465.
- ^ Bateman RM, WA DiMichele. (2002). Generating and filtering major phenotypic novelties: neoGoldschmidtian saltation revisited. pp. 109–159 in QCB Cronk, RM Bateman, JA Hawkins, eds. Developmental genetics and plant evolution. Taylor & Francis, London
- ^ Louis P. Ronse De Craene. (2002). The Evolutionary Significance of Homeosis in Flowers: A Morphological Perspective. International Journal of Plant Sciences. Vol. 164, No. 5, Flowers—Diversity, Development, and Evolution A conference organized and held at the Institute of Systematic Botany, University of Zurich, Switzerland, July 5–7. pp. 225-235.
- ^ France Dufresne, Paul D. N. Herbert (1994). "Hybridization and origins of polyploidy". Proceedings of the Royal Society. Retrieved 2010-05-06.
- ^ Eldredge, Niles and S. J. Gould (1972). "Punctuated equilibria: an alternative to phyletic gradualism" In T.J.M. Schopf, ed., Models in Paleobiology. San Francisco: Freeman Cooper. pp. 82-115.
- ^ Stephen Jay Gould. (1981). Evolution as Fact and Theory. Discover 2 (May): 34-37.
- ^ Steven M. Stanley. (1981). The New Evolutionary Timetable. New York: Basic Books. p. 135
Further reading
- Thomas C. Baker. (2002). Mechanism for saltational shifts in pheromone communication systems. Proceedings of the National Academy of Sciences. USA 99. 13368-13370.
- Richard M. Bateman, William A. DiMichele. (2002). Generating and filtering major phenotypic novelties: neoGoldschmidtian saltation revisited. In: Cronk QCB, Bateman RM, Hawkins JA, eds. Developmental genetics and plant evolution. London: Taylor & Francis. pp. 109–159.
- Brian K. Hall, Roy D. Pearson, Gerd B. Müller. (2004). Environment, Development, and Evolution: Toward a Synthesis. MIT Press. ISBN 978-0262083195
- Ulrich Kutschera, Karl J. Niklas. (2008). Macroevolution via secondary endosymbiosis: a Neo-Goldschmidtian view of unicellular hopeful monsters and Darwin's primordial intermediate form. Theory in Biosciences 127: 277-289.
- David J. Merrell. (1994). The Adaptive Seascape: The Mechanism of Evolution. University of Minnesota Press. ISBN 978-0816623488
- Jeffrey H. Schwartz. (2006). Sudden origins: a general mechanism of evolution based on stress protein concentration and rapid environmental change. The Anatomical Record. 289: 38–46.
- Gamberale-Stille G, Balogh AC, Tullberg BS, Leimar O. (2012). Feature saltation and the evolution of mimicry. Evolution 66: 807-17.
- Guenter Theissen. (2009). Saltational evolution: hopeful monsters are here to stay. Theory in Bioscience. 128, 43-51.
