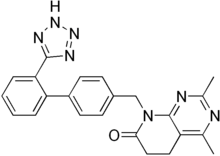
Tasosartan
Appearance
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H21N7O |
| Molar mass | 411.459 g/mol g·mol−1 |
| | |
Tasosartan is an angiotensin II receptor antagonist.
It was withdrawn from FDA review by the manufacturer after phase III clinical trials showed elevated transaminases (a sign of possible liver toxicity) in a significant number of participants given the drug.[1][2]
References
- ^ Atkinson AJ, et al. (2007). Principles of clinical pharmacology. Amsterdam: Elsevier. p. 515. ISBN 0-12-369417-5.
- ^ Dina R, Jafari M (July 2000). "Angiotensin II-receptor antagonists: an overview". Am J Health Syst Pharm. 57 (13): 1231–41. PMID 10902066.
