Thallium(I) carbonate
Appearance
(Redirected from Thallium carbonate)
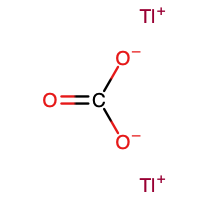
| |
| Names | |
|---|---|
| Other names
thallium monocarbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.026.759 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1707 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Tl2CO3 | |
| Molar mass | 468.776 g/mol |
| Appearance | white crystals |
| Odor | odorless |
| Density | 7.11 g/cm3, solid |
| Melting point | 272 °C (522 °F; 545 K) |
| 5.2 g/100 mL (25 °C) 27.2 g/100 mL (100 °C) | |
| Solubility | insoluble in alcohol, ether, acetone |
| −101.6·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| Hazards | |
| GHS labelling:[2] | |
  
| |
| Danger | |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P330, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
21 mg/kg (mouse, oral)[1] |
LDLo (lowest published)
|
23 mg/kg (rat, oral)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thallium(I) carbonate is the inorganic compound with the formula Tl2CO3. It is a white, water-soluble salt. It has no or very few commercial applications. It is produced by treatment of thallous hydroxide with CO2.[3]
Safety
[edit]Like other thallium compounds, it is extremely toxic, with an oral median lethal dose of 21 mg/kg in mice. Due to its toxicity, it is listed in the United States List of Extremely Hazardous Substances as of 2007.[4]
References
[edit]- ^ a b "Thallium (soluble compounds, as Tl)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Thallous carbonate". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Micke, Heinrich; Wolf, Hans Uwe (2000). "Thallium and Thallium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_607. ISBN 978-3527306732.
- ^ "Emergency First Aid Treatment Guide THALLOUS CARBONATE". Chemical Emergency Preparedness and Prevention. U.S. Environmental Protection Agency. Retrieved 2 June 2012.

