Beryllium hydride

| |
| Names | |
|---|---|
| Other names
Beryllium dihydride
Beryllium hydride Beryllane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BeH2 | |
| Molar mass | 11.03 g mol−1 |
| Appearance | white solid[1] |
| Density | 0.65 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) decomposes[1] |
| decomposes | |
| Solubility | insoluble in diethyl ether, toluene |
| Thermochemistry | |
Heat capacity (C)
|
30.124 J/mol K |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[2] |
REL (Recommended)
|
Ca C 0.0005 mg/m3 (as Be)[2] |
IDLH (Immediate danger)
|
Ca [4 mg/m3 (as Be)][2] |
| Related compounds | |
Other cations
|
lithium hydride, sodium hydride, magnesium hydride, calcium hydride, boron hydrides, aluminium hydride |
Related compounds
|
beryllium fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Beryllium hydride (systematically named poly[beryllane(2)] and beryllium dihydride) is an inorganic compound with the chemical formula (BeH
2)n (also written ([BeH
2])n or BeH
2). This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it. Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded[1] (three-center two-electron bond).
Synthesis
[edit]Unlike the other group 2 metals, beryllium does not react with hydrogen.[3] Instead, BeH2 is prepared from preformed beryllium(II) compounds. It was first synthesized in 1951 by treating dimethylberyllium, Be(CH3)2, with lithium aluminium hydride, LiAlH4.[4]
Purer BeH2 forms from the pyrolysis of di-tert-butylberyllium, Be(C[CH3]3)2 at 210°C.[5]
A route to highly pure samples involves the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH4)2:[1]
- Be(BH4)2 + 2 PPh3 → BeH2 + 2 Ph3PBH3
Structure
[edit]Gaseous form
[edit]
Isolated molecules of BeH
2 (sometimes called dihydridoberyllium and written [BeH
2] to emphasize the differences with the solid state) are only stable as a dilute gas. When condensed, unsolvated BeH
2 will spontaneously autopolymerise.
Free molecular BeH2 produced by high-temperature electrical discharge has been confirmed to have linear geometry with a Be-H bond length of 133.376 pm. Its hybridization is sp.[6]
Condensed Beryllium hydride
[edit]BeH2 is usually formed as an amorphous white solid, but a hexagonal crystalline form with a higher density (~0.78 g/cm3) was reported,[7] prepared by heating amorphous BeH2 under pressure, with 0.5-2.5% LiH as a catalyst.
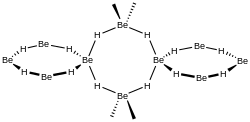
A more recent investigation found that crystalline beryllium hydride has a body-centred orthorhombic unit cell, containing a network of corner-sharing BeH4 tetrahedra, in contrast to the flat, hydrogen-bridged, infinite chains previously thought to exist in crystalline BeH2.[8]
Studies of the amorphous form also find that it consists of a network of corner shared tetrahedra.[9]
Chemical properties
[edit]Reaction with water and acids
[edit]Beryllium hydride reacts slowly with water but is rapidly hydrolysed by acid such as hydrogen chloride to form beryllium chloride.[3]
- BeH2 + 2 H2O → Be(OH)2 + 2 H2
- BeH2 + 2 HCl → BeCl2 + 2 H2
Reaction with Lewis bases
[edit]The two-coordinate hydridoberyllium group can accept an electron-pair donating ligand (L) into the molecule by adduction:[10]
- [BeH
2] + L → [BeH
2L]
Because these reactions are energetically favored, beryllium hydride has Lewis-acidic character.
The reaction with lithium hydride (in which the hydride ion is the Lewis base), forms sequentially LiBeH3 and Li2BeH4.[3] The latter contains the tetrahydridoberyllate(2-) anion BeH2−
4.
Beryllium hydride reacts with trimethylamine, N(CH3)3 to form a dimeric adduct with bridging hydrides.[11] However, with dimethylamine, HN(CH3)2 it forms a trimeric beryllium diamide, [Be(N(CH3)2)2]3, and hydrogen.[3]
References
[edit]- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 115. ISBN 978-0-08-037941-8.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5, p. 1048
- ^ Glenn D. Barbaras; Clyde Dillard; A. E. Finholt; Thomas Wartik; K. E. Wilzbach & H. I. Schlesinger (1951). "The Preparation of the Hydrides of Zinc, Cadmium, Beryllium, Magnesium and Lithium by the Use of Lithium Aluminum Hydride". Journal of the American Chemical Society. 73 (10): 4585–4590. doi:10.1021/ja01154a025.
- ^ G. E. Coates & F. Glockling (1954). "Di-tert.-butylberyllium and beryllium hydride". Journal of the Chemical Society: 2526–2529. doi:10.1039/JR9540002526.
- ^ Peter F. Bernath; Alireza Shayesteh; Keith Tereszchuk; Reginald Colin (2002). "The Vibration-Rotation Emission Spectrum of Free BeH2". Science. 297 (5585): 1323–1324. Bibcode:2002Sci...297.1323B. doi:10.1126/science.1074580. PMID 12193780. S2CID 40961746.
- ^ G. J. Brendel; E. M. Marlett & L. M. Niebylski (1978). "Crystalline beryllium hydride". Inorganic Chemistry. 17 (12): 3589–3592. doi:10.1021/ic50190a051.
- ^ a b Gordon S. Smith; Quintin C. Johnson; Deane K. Smith; D. E. Cox; Robert L. Snyder; Rong-Sheng Zhou & Allan Zalkin (1988). "The crystal and molecular structure of beryllium hydride". Solid State Communications. 67 (5): 491–494. Bibcode:1988SSCom..67..491S. doi:10.1016/0038-1098(84)90168-6.
- ^ Sujatha Sampath; Kristina M. Lantzky; Chris J. Benmore; Jörg Neuefeind & Joan E. Siewenie (2003). "Structural quantum isotope effects in amorphous beryllium hydride". J. Chem. Phys. 119 (23): 12499. Bibcode:2003JChPh.11912499S. doi:10.1063/1.1626638.
- ^ Sharp, Stephanie B.; Gellene, Gregory I. (23 November 2000). "σ Bond Activation by Cooperative Interaction with ns2 Atoms: Be + n H
2, n = 1−3". The Journal of Physical Chemistry A. 104 (46): 10951–10957. doi:10.1021/jp002313m. - ^ Shepherd Jr., Lawrence H.; Ter Haar, G. L.; Marlett, Everett M. (April 1969). "Amine complexes of beryllium hydride". Inorganic Chemistry. 8 (4): 976–979. doi:10.1021/ic50074a051.
