Actinidain: Difference between revisions
Content deleted Content added
Bluelinking 1 books for verifiability.) #IABot (v2.1alpha3 |
Citation bot (talk | contribs) Alter: journal, url. Add: url, chapter-url. Removed or converted URL. Some additions/deletions were actually parameter name changes. | You can use this bot yourself. Report bugs here. | Activated by Headbomb | via #UCB_webform |
||
| Line 10: | Line 10: | ||
| caption = Biological assembly image of actinidain from ''[[Actinidia chinensis]]''. From {{PDB|1AEC}}. |
| caption = Biological assembly image of actinidain from ''[[Actinidia chinensis]]''. From {{PDB|1AEC}}. |
||
}} |
}} |
||
'''Actinidain''' ({{EC number|3.4.22.14}}, ''actinidin'', ''Actinidia anionic protease'', ''proteinase A2 of Actinidia chinensis'') is a type of [[cysteine protease]] enzyme found in fruits including [[kiwifruit]] (genus ''[[Actinidia]]''), [[pineapple]], [[mango]], [[banana]] and [[papaya]]. This enzyme is part of the [[papain]]-like [[peptidase]] C1 family.<ref>{{cite journal | vauthors = Baker EN, Boland MJ, Calder PC, Hardman MJ | title = The specificity of actinidin and its relationship to the structure of the enzyme | journal = Biochimica et Biophysica Acta | volume = 616 | issue = 1 | pages = 30–4 | date = November 1980 | pmid = 7002215 | doi = 10.1016/0005-2744(80)90260-0 }}</ref><ref>{{cite journal | vauthors = Kamphuis IG, Drenth J, Baker EN | title = Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain | journal = Journal of Molecular Biology | volume = 182 | issue = 2 | pages = 317–29 | date = March 1985 | pmid = 3889350 | doi = 10.1016/0022-2836(85)90348-1 }}</ref><ref>{{cite book |chapter = The thiol proteases: structure and mechanism |title = Active Sites of Enzymes |vauthors = Baker EN, Drenth J |year = 1987 |series = Biological Macromolecules and Assemblies |volume = 3 |pages = [https://archive.org/details/biologicalmacrom0000unse/page/314 314–368] |veditors = Jurnak FA, McPherson A |edition = |publisher = John Wiley and Sons |location = New York |isbn = 978-0-471-85142-4 |url = https://archive.org/details/biologicalmacrom0000unse/page/314 }}</ref><ref>{{cite journal | vauthors = Gul S, Mellor GW, Thomas EW, Brocklehurst K | title = Temperature-dependences of the kinetics of reactions of papain and actinidin with a series of reactivity probes differing in key molecular recognition features | journal = The Biochemical Journal | volume = 396 | issue = 1 | pages = 17–21 | date = May 2006 | pmid = 16445383 | pmc = 1449998 | doi = 10.1042/BJ20051501 }}</ref> |
'''Actinidain''' ({{EC number|3.4.22.14}}, ''actinidin'', ''Actinidia anionic protease'', ''proteinase A2 of Actinidia chinensis'') is a type of [[cysteine protease]] enzyme found in fruits including [[kiwifruit]] (genus ''[[Actinidia]]''), [[pineapple]], [[mango]], [[banana]] and [[papaya]]. This enzyme is part of the [[papain]]-like [[peptidase]] C1 family.<ref>{{cite journal | vauthors = Baker EN, Boland MJ, Calder PC, Hardman MJ | title = The specificity of actinidin and its relationship to the structure of the enzyme | journal = Biochimica et Biophysica Acta (BBA) - Enzymology | volume = 616 | issue = 1 | pages = 30–4 | date = November 1980 | pmid = 7002215 | doi = 10.1016/0005-2744(80)90260-0 }}</ref><ref>{{cite journal | vauthors = Kamphuis IG, Drenth J, Baker EN | title = Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain | journal = Journal of Molecular Biology | volume = 182 | issue = 2 | pages = 317–29 | date = March 1985 | pmid = 3889350 | doi = 10.1016/0022-2836(85)90348-1 }}</ref><ref>{{cite book |chapter = The thiol proteases: structure and mechanism |title = Active Sites of Enzymes |vauthors = Baker EN, Drenth J |year = 1987 |series = Biological Macromolecules and Assemblies |volume = 3 |pages = [https://archive.org/details/biologicalmacrom0000unse/page/314 314–368] |veditors = Jurnak FA, McPherson A |edition = |publisher = John Wiley and Sons |location = New York |isbn = 978-0-471-85142-4 |chapter-url = https://archive.org/details/biologicalmacrom0000unse/page/314 }}</ref><ref>{{cite journal | vauthors = Gul S, Mellor GW, Thomas EW, Brocklehurst K | title = Temperature-dependences of the kinetics of reactions of papain and actinidin with a series of reactivity probes differing in key molecular recognition features | journal = The Biochemical Journal | volume = 396 | issue = 1 | pages = 17–21 | date = May 2006 | pmid = 16445383 | pmc = 1449998 | doi = 10.1042/BJ20051501 }}</ref> |
||
As a known [[allergen]] in kiwifruit,<ref>{{cite journal | vauthors = Maddumage R, Nieuwenhuizen NJ, Bulley SM, Cooney JM, Green SA, Atkinson RG | title = Diversity and relative levels of actinidin, kiwellin, and thaumatin-like allergens in 15 varieties of kiwifruit (Actinidia) | journal = Journal of Agricultural and Food Chemistry | volume = 61 | issue = 3 | pages = 728–39 | date = January 2013 | pmid = 23289429 | doi = 10.1021/jf304289f }}</ref> the enzyme is under preliminary research for its effect on [[tight junction]] proteins of intestinal [[epithelial cell]]s.<ref>{{cite journal | vauthors = Grozdanovic MM, Čavić M, Nešić A, Andjelković U, Akbari P, Smit JJ, Gavrović-Jankulović M | title = Kiwifruit cysteine protease actinidin compromises the intestinal barrier by disrupting tight junctions | journal = Biochimica et Biophysica Acta | volume = 1860 | issue = 3 | pages = 516–26 | date = March 2016 | pmid = 26701113 | doi = 10.1016/j.bbagen.2015.12.005 }}</ref><ref>{{cite journal | vauthors = Cavic M, Grozdanovic MM, Bajic A, Jankovic R, Andjus PR, Gavrovic-Jankulovic M | title = The effect of kiwifruit (Actinidia deliciosa) cysteine protease actinidin on the occludin tight junction network in T84 intestinal epithelial cells | journal = Food and Chemical Toxicology | volume = 72 | pages = 61–8 | date = October 2014 | pmid = 25042511 | doi = 10.1016/j.fct.2014.07.012 }}</ref> |
As a known [[allergen]] in kiwifruit,<ref>{{cite journal | vauthors = Maddumage R, Nieuwenhuizen NJ, Bulley SM, Cooney JM, Green SA, Atkinson RG | title = Diversity and relative levels of actinidin, kiwellin, and thaumatin-like allergens in 15 varieties of kiwifruit (Actinidia) | journal = Journal of Agricultural and Food Chemistry | volume = 61 | issue = 3 | pages = 728–39 | date = January 2013 | pmid = 23289429 | doi = 10.1021/jf304289f }}</ref> the enzyme is under preliminary research for its effect on [[tight junction]] proteins of intestinal [[epithelial cell]]s.<ref>{{cite journal | vauthors = Grozdanovic MM, Čavić M, Nešić A, Andjelković U, Akbari P, Smit JJ, Gavrović-Jankulović M | title = Kiwifruit cysteine protease actinidin compromises the intestinal barrier by disrupting tight junctions | journal = Biochimica et Biophysica Acta (BBA) - General Subjects | volume = 1860 | issue = 3 | pages = 516–26 | date = March 2016 | pmid = 26701113 | doi = 10.1016/j.bbagen.2015.12.005 | url = http://cer.ihtm.bg.ac.rs/handle/123456789/3154 }}</ref><ref>{{cite journal | vauthors = Cavic M, Grozdanovic MM, Bajic A, Jankovic R, Andjus PR, Gavrovic-Jankulovic M | title = The effect of kiwifruit (Actinidia deliciosa) cysteine protease actinidin on the occludin tight junction network in T84 intestinal epithelial cells | journal = Food and Chemical Toxicology | volume = 72 | pages = 61–8 | date = October 2014 | pmid = 25042511 | doi = 10.1016/j.fct.2014.07.012 }}</ref> |
||
Actinidain is commercially useful as a meat tenderiser<ref>{{cite journal | vauthors = Bekhit AA, Hopkins DL, Geesink G, Bekhit AA, Franks P | title = Exogenous proteases for meat tenderization | journal = Critical Reviews in Food Science and Nutrition | volume = 54 | issue = 8 | pages = 1012–31 | year = 2014 | pmid = 24499119 | doi = 10.1080/10408398.2011.623247 }}</ref><ref name=":0">{{cite journal | vauthors = Eshamah H, Han I, Naas H, Acton J, Dawson P | title = Antibacterial effects of natural tenderizing enzymes on different strains of Escherichia coli O157:H7 and Listeria monocytogenes on beef | journal = Meat Science | volume = 96 | issue = 4 | pages = 1494–500 | date = April 2014 | pmid = 24447905 | doi = 10.1016/j.meatsci.2013.12.010 }}</ref> and in coagulating milk for dairy products.<ref>{{cite journal | last1 = Katsaros | first1 = George I.| last2 = Tavantzis | first2 = George | last3 = Taoukis | first3 = Petros S. | name-list-format = vanc | date = January 2010 | title = Production of novel dairy products using actinidin and high pressure as enzyme activity regulator | journal = Innovative Food Science & Emerging Technologies | volume = 11 | issue = 1 | pages = 47–51 | doi = 10.1016/j.ifset.2009.08.007 }}</ref> The denaturation temperature of actinidain is {{cvt|60|C|F}}, lower than that of similar meat tenderising enzymes [[bromelain]] from [[pineapple]] and [[papain]] from [[papaya]].<ref>{{cite book | first = Rodrigo | last =Tarté | name-list-format = vanc | title = Ingredients in meat products properties, functionality and applications | date = 2008 | publisher = Springer | location = New York | isbn = 978-0-387-71327-4 | url = https://books.google.com/?id=C-wrQaaXxj0C&pg=PA179&lpg=PA179&dq=actinidin+denaturation+temperature#v=onepage |
Actinidain is commercially useful as a meat tenderiser<ref>{{cite journal | vauthors = Bekhit AA, Hopkins DL, Geesink G, Bekhit AA, Franks P | title = Exogenous proteases for meat tenderization | journal = Critical Reviews in Food Science and Nutrition | volume = 54 | issue = 8 | pages = 1012–31 | year = 2014 | pmid = 24499119 | doi = 10.1080/10408398.2011.623247 }}</ref><ref name=":0">{{cite journal | vauthors = Eshamah H, Han I, Naas H, Acton J, Dawson P | title = Antibacterial effects of natural tenderizing enzymes on different strains of Escherichia coli O157:H7 and Listeria monocytogenes on beef | journal = Meat Science | volume = 96 | issue = 4 | pages = 1494–500 | date = April 2014 | pmid = 24447905 | doi = 10.1016/j.meatsci.2013.12.010 }}</ref> and in coagulating milk for dairy products.<ref>{{cite journal | last1 = Katsaros | first1 = George I.| last2 = Tavantzis | first2 = George | last3 = Taoukis | first3 = Petros S. | name-list-format = vanc | date = January 2010 | title = Production of novel dairy products using actinidin and high pressure as enzyme activity regulator | journal = Innovative Food Science & Emerging Technologies | volume = 11 | issue = 1 | pages = 47–51 | doi = 10.1016/j.ifset.2009.08.007 }}</ref> The denaturation temperature of actinidain is {{cvt|60|C|F}}, lower than that of similar meat tenderising enzymes [[bromelain]] from [[pineapple]] and [[papain]] from [[papaya]].<ref>{{cite book | first = Rodrigo | last =Tarté | name-list-format = vanc | title = Ingredients in meat products properties, functionality and applications | date = 2008 | publisher = Springer | location = New York | isbn = 978-0-387-71327-4 | url = https://books.google.com/books?id=C-wrQaaXxj0C&pg=PA179&lpg=PA179&dq=actinidin+denaturation+temperature#v=onepage}}</ref> |
||
== References == |
== References == |
||
Revision as of 23:59, 24 May 2020
| actinidain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
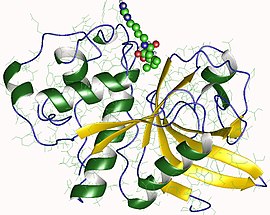 Biological assembly image of actinidain from Actinidia chinensis. From PDB: 1AEC. | |||||||||
| Identifiers | |||||||||
| EC no. | 3.4.22.14 | ||||||||
| CAS no. | 39279-27-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Actinidain (EC 3.4.22.14, actinidin, Actinidia anionic protease, proteinase A2 of Actinidia chinensis) is a type of cysteine protease enzyme found in fruits including kiwifruit (genus Actinidia), pineapple, mango, banana and papaya. This enzyme is part of the papain-like peptidase C1 family.[1][2][3][4]
As a known allergen in kiwifruit,[5] the enzyme is under preliminary research for its effect on tight junction proteins of intestinal epithelial cells.[6][7]
Actinidain is commercially useful as a meat tenderiser[8][9] and in coagulating milk for dairy products.[10] The denaturation temperature of actinidain is 60 °C (140 °F), lower than that of similar meat tenderising enzymes bromelain from pineapple and papain from papaya.[11]
References
- ^ Baker EN, Boland MJ, Calder PC, Hardman MJ (November 1980). "The specificity of actinidin and its relationship to the structure of the enzyme". Biochimica et Biophysica Acta (BBA) - Enzymology. 616 (1): 30–4. doi:10.1016/0005-2744(80)90260-0. PMID 7002215.
- ^ Kamphuis IG, Drenth J, Baker EN (March 1985). "Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain". Journal of Molecular Biology. 182 (2): 317–29. doi:10.1016/0022-2836(85)90348-1. PMID 3889350.
- ^ Baker EN, Drenth J (1987). "The thiol proteases: structure and mechanism". In Jurnak FA, McPherson A (eds.). Active Sites of Enzymes. Biological Macromolecules and Assemblies. Vol. 3. New York: John Wiley and Sons. pp. 314–368. ISBN 978-0-471-85142-4.
- ^ Gul S, Mellor GW, Thomas EW, Brocklehurst K (May 2006). "Temperature-dependences of the kinetics of reactions of papain and actinidin with a series of reactivity probes differing in key molecular recognition features". The Biochemical Journal. 396 (1): 17–21. doi:10.1042/BJ20051501. PMC 1449998. PMID 16445383.
- ^ Maddumage R, Nieuwenhuizen NJ, Bulley SM, Cooney JM, Green SA, Atkinson RG (January 2013). "Diversity and relative levels of actinidin, kiwellin, and thaumatin-like allergens in 15 varieties of kiwifruit (Actinidia)". Journal of Agricultural and Food Chemistry. 61 (3): 728–39. doi:10.1021/jf304289f. PMID 23289429.
- ^ Grozdanovic MM, Čavić M, Nešić A, Andjelković U, Akbari P, Smit JJ, Gavrović-Jankulović M (March 2016). "Kiwifruit cysteine protease actinidin compromises the intestinal barrier by disrupting tight junctions". Biochimica et Biophysica Acta (BBA) - General Subjects. 1860 (3): 516–26. doi:10.1016/j.bbagen.2015.12.005. PMID 26701113.
- ^ Cavic M, Grozdanovic MM, Bajic A, Jankovic R, Andjus PR, Gavrovic-Jankulovic M (October 2014). "The effect of kiwifruit (Actinidia deliciosa) cysteine protease actinidin on the occludin tight junction network in T84 intestinal epithelial cells". Food and Chemical Toxicology. 72: 61–8. doi:10.1016/j.fct.2014.07.012. PMID 25042511.
- ^ Bekhit AA, Hopkins DL, Geesink G, Bekhit AA, Franks P (2014). "Exogenous proteases for meat tenderization". Critical Reviews in Food Science and Nutrition. 54 (8): 1012–31. doi:10.1080/10408398.2011.623247. PMID 24499119.
- ^ Eshamah H, Han I, Naas H, Acton J, Dawson P (April 2014). "Antibacterial effects of natural tenderizing enzymes on different strains of Escherichia coli O157:H7 and Listeria monocytogenes on beef". Meat Science. 96 (4): 1494–500. doi:10.1016/j.meatsci.2013.12.010. PMID 24447905.
- ^ Katsaros, George I.; Tavantzis, George; Taoukis, Petros S. (January 2010). "Production of novel dairy products using actinidin and high pressure as enzyme activity regulator". Innovative Food Science & Emerging Technologies. 11 (1): 47–51. doi:10.1016/j.ifset.2009.08.007.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Tarté, Rodrigo (2008). Ingredients in meat products properties, functionality and applications. New York: Springer. ISBN 978-0-387-71327-4.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
External links
- The MEROPS online database for peptidases and their inhibitors: C01.007
- EC 3.4.22.14
- actinidain at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
