Hexane

| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexane[2] | |
| Other names
Sextane,[1] hexacarbane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1730733 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.435 |
| EC Number |
|
| 1985 | |
| KEGG | |
| MeSH | n-hexane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1208 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H14 | |
| Molar mass | 86.178 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Petrolic |
| Density | 0.6606 g mL−1[3] |
| Melting point | −96 to −94 °C; −141 to −137 °F; 177 to 179 K |
| Boiling point | 68.5 to 69.1 °C; 155.2 to 156.3 °F; 341.6 to 342.2 K |
| 9.5 mg L−1 | |
| log P | 3.764 |
| Vapor pressure | 17.60 kPa (at 20.0 °C) |
Henry's law
constant (kH) |
7.6 nmol Pa−1 kg−1 |
| UV-vis (λmax) | 200 nm |
| −74.6·10−6 cm3/mol | |
Refractive index (nD)
|
1.375 |
| Viscosity | 0.3 mPa·s |
| 0.08 D | |
| Thermochemistry | |
Heat capacity (C)
|
265.2 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
296.06 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−199.4–−198.0 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−4180–−4140 kJ mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Reproductive toxicity – After aspiration, pulmonary oedema, pneumonitis[4] |
| GHS labelling: | |
   
| |
| Danger | |
| H225, H302, H305, H315, H336, H361fd, H373, H411 | |
| P201, P202, P210, P233, P235, P240, P241, P242, P243, P260, P264, P271, P273, P280, P281, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P308+P313, P310, P312, P314, P332+P313, P363, P370+P378, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | −26.0 °C (−14.8 °F; 247.2 K) |
| 234.0 °C (453.2 °F; 507.1 K) | |
| Explosive limits | 1.2–7.7% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
25 g kg−1 (oral, rat) 28710 mg/kg (rat, oral)[6] |
LDLo (lowest published)
|
56137 mg/kg (rat, oral)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 500 ppm (1800 mg/m3)[5] |
REL (Recommended)
|
TWA 50 ppm (180 mg/m3)[5] |
IDLH (Immediate danger)
|
1100 ppm[5] |
| Related compounds | |
Related alkanes
|
|
| Supplementary data page | |
| Hexane (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexane (/ˈhɛkseɪn/) or n-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.[7]
Hexane is a colorless liquid, odorless when pure, and with a boiling point of approximately 69 °C (156 °F). It is widely used as a cheap, relatively safe, largely unreactive, and easily evaporated non-polar solvent, and modern gasoline blends contain about 3% hexane.[8]
The term hexanes refers to a mixture, composed largely (>60%) of n-hexane, with varying amounts of the isomeric compounds 2-methylpentane and 3-methylpentane, and possibly, smaller amounts of nonisomeric C5, C6, and C7 (cyclo)alkanes. These "hexanes" mixtures are cheaper than pure hexane and are often used in large-scale operations not requiring a single isomer (e.g., as cleaning solvent or for chromatography).
Isomers[edit]
| Common name | IUPAC name | Text formula | Skeletal formula |
|---|---|---|---|
| Normal hexane, n-Hexane |
Hexane | CH3(CH2)4CH3 | |
| Isohexane | 2-Methylpentane | (CH3)2CH(CH2)2CH3 | 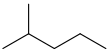
|
| 3-Methylpentane | CH3CH2CH(CH3)CH2CH3 | 
| |
| 2,3-Dimethylbutane | (CH3)2CHCH(CH3)2 | 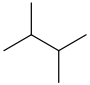
| |
| Neohexane | 2,2-Dimethylbutane | (CH3)3CCH2CH3 | 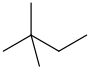
|
Uses[edit]
In industry, hexanes are used in the formulation of glues for shoes, leather products, and roofing. They are also used to extract cooking oils (such as canola oil or soy oil) from seeds, for cleansing and degreasing a variety of items, and in textile manufacturing. They are commonly used in food based soybean oil extraction in the United States, and are potentially present as contaminants in all soy food products in which the technique is used[citation needed]; the lack of regulation by the FDA of this contaminant is a matter of some controversy.[9][10]
A typical laboratory use of hexanes is to extract oil and grease contaminants from water and soil for analysis.[11] Since hexane cannot be easily deprotonated, it is used in the laboratory for reactions that involve very strong bases, such as the preparation of organolithiums. For example, butyllithiums are typically supplied as a hexane solution.[12]
Hexanes are commonly used in chromatography as a non-polar solvent. Higher alkanes present as impurities in hexanes have similar retention times as the solvent, meaning that fractions containing hexane will also contain these impurities. In preparative chromatography, concentration of a large volume of hexanes can result in a sample that is appreciably contaminated by alkanes. This may result in a solid compound being obtained as an oil and the alkanes may interfere with analysis.
Production[edit]
Hexane is chiefly obtained by refining crude oil. The exact composition of the fraction depends largely on the source of the oil (crude or reformed) and the constraints of the refining.[13] The industrial product (usually around 50% by weight of the straight-chain isomer) is the fraction boiling at 65–70 °C (149–158 °F).
Physical properties[edit]
All alkanes are colorless.[14][15] The boiling points of the various hexanes are somewhat similar and, as for other alkanes, are generally lower for the more branched forms. The melting points are quite different and the trend is not apparent.[16]
| Isomer | M.P. (°C) | M.P. (°F) | B.P. (°C) | B.P. (°F) |
|---|---|---|---|---|
| n-hexane | −95.3 | −139.5 | 68.7 | 155.7 |
| 3-methylpentane | −118.0 | −180.4 | 63.3 | 145.9 |
| 2-methylpentane (isohexane) | −153.7 | −244.7 | 60.3 | 140.5 |
| 2,3-dimethylbutane | −128.6 | −199.5 | 58.0 | 136.4 |
| 2,2-dimethylbutane (neohexane) | −99.8 | −147.6 | 49.7 | 121.5 |
Hexane has considerable vapor pressure at room temperature:
| Temperature (°C) | Temperature (°F) | Vapor pressure (mmHg) | Vapor pressure (kPa) |
|---|---|---|---|
| −40 | −40 | 3.36 | 0.448 |
| −30 | −22 | 7.12 | 0.949 |
| −20 | −4 | 14.01 | 1.868 |
| −10 | 14 | 25.91 | 3.454 |
| 0 | 32 | 45.37 | 6.049 |
| 10 | 50 | 75.74 | 10.098 |
| 20 | 68 | 121.26 | 16.167 |
| 25 | 77 | 151.28 | 20.169 |
| 30 | 86 | 187.11 | 24.946 |
| 40 | 104 | 279.42 | 37.253 |
| 50 | 122 | 405.31 | 54.037 |
| 60 | 140 | 572.76 | 76.362 |
Reactivity[edit]
Like most alkanes, hexanes characteristically exhibit low reactivity and are suitable solvents for reactive compounds. Commercial samples of n-hexane however often contains methylcyclopentane, which features tertiary C-H bonds, which are incompatible with some radical reactions.[17]
Safety[edit]
Inhalation of n-hexane at 5000 ppm for 10 minutes produces marked vertigo; 2500-1000 ppm for 12 hours produces drowsiness, fatigue, loss of appetite, and paresthesia in the distal extremities; 2500–5000 ppm produces muscle weakness, cold pulsation in the extremities, blurred vision, headache, and anorexia.[18] Chronic occupational exposure to elevated levels of n-hexane has been demonstrated to be associated with peripheral neuropathy in auto mechanics in the US, and neurotoxicity in workers in printing presses, and shoe and furniture factories in Asia, Europe, and North America.[19]
The US National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) for hexane isomers (not n-hexane) of 100 ppm (350 mg/m3 (0.15 gr/cu ft)) over an 8-hour workday.[20] However, for n-hexane, the current NIOSH REL is 50 ppm (180 mg/m3 (0.079 gr/cu ft)) over an 8-hour workday.[21] This limit was proposed as a permissible exposure limit (PEL) by the Occupational Safety and Health Administration in 1989; however, this PEL was overruled in US courts in 1992.[22] The current n-hexane PEL in the US is 500 ppm (1,800 mg/m3 (0.79 gr/cu ft)).[21]
Hexane and other volatile hydrocarbons (petroleum ether) present an aspiration risk.[23] n-Hexane is sometimes used as a denaturant for alcohol, and as a cleaning agent in the textile, furniture, and leather industries. It is slowly being replaced with other solvents.[24]
Like gasoline, hexane is highly volatile and is an explosion risk. The 1981 Louisville sewer explosions, which destroyed over 13 mi (21 km) of sewer lines and streets in Kentucky, were caused by ignition of hexane vapors which had been illegally discharged from a soybean processing plant owned by Ralston-Purina. Hexane was attributed as the cause of an explosion that occurred in the National University of Río Cuarto, Argentina on 5 December 2007, due to a hexane spill near a heat-producing machine that exploded, producing a fire that killed one student and injured 24 more.
Incidents[edit]
Occupational hexane poisoning has occurred with Japanese sandal workers, Italian shoe workers,[25] Taiwan press proofing workers, and others.[26] Analysis of Taiwanese workers has shown occupational exposure to substances including n-hexane.[27] In 2010–2011, Chinese workers manufacturing iPhones were reported to have suffered hexane poisoning.[28][29]
Biotransformation[edit]
n-Hexane is biotransformed to 2-hexanol and further to 2,5-Hexanediol in the body. The conversion is catalyzed by the enzyme cytochrome P450 utilizing oxygen from air. 2,5-Hexanediol may be further oxidized to 2,5-hexanedione, which is neurotoxic and produces a polyneuropathy.[24] In view of this behavior, replacement of n-hexane as a solvent has been discussed. n-Heptane is a possible alternative.[30]
See also[edit]
References[edit]
- ^ Hofmann, August Wilhelm Von (1 January 1867). "I. On the action of trichloride of phosphorus on the salts of the aromatic monamines". Proceedings of the Royal Society of London. 15: 54–62. doi:10.1098/rspl.1866.0018. S2CID 98496840.
- ^ "n-hexane – Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Archived from the original on 8 March 2012. Retrieved 31 December 2011.
- ^ William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. pp. 3–298. ISBN 978-1-4987-5429-3.
- ^ GHS Classification on [PubChem]
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0322". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "n-Hexane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ PubChem. "n-HEXANE". pubchem.ncbi.nlm.nih.gov. Retrieved 3 November 2023.
- ^ "n-Hexane - Hazardous Agents". Haz-Map. Retrieved 7 July 2022.
- ^ "The Tofurky Company : Our Ingredients". Tofurky.com. Archived from the original on 17 March 2015. Retrieved 17 March 2015.
- ^ Palmer, Brian (26 April 2010). "A study found hexane in soy protein. Should you stop eating veggie burgers?". Slate.com. Archived from the original on 9 March 2015. Retrieved 17 March 2015.
- ^ Use of ozone depleting substances in laboratories. Kbh: Nordisk Ministerråd. 2003. ISBN 92-893-0884-2. OCLC 474188215. Archived from the original on 16 July 2012.
- ^ Schwindeman, James A.; Woltermann, Chris J.; Letchford, Robert J. (1 May 2002). "Safe handling of organolithium compounds in the laboratory". Chemical Health & Safety. 9 (3): 6–11. doi:10.1016/s1074-9098(02)00295-2. ISSN 1074-9098.
- ^ Le Van Mao, R.; Melancon, S.; Gauthier-Campbell, C.; Kletnieks, P. (1 May 2001). "Selective deep catalytic cracking process (SDCC) of petroleum feedstocks for the production of light olefins. I. The Catlever effect obtained with a two reaction-zones system on the conversion of n-hexane". Catalysis Letters. 73 (2): 181–186. doi:10.1023/A:1016685523095. ISSN 1572-879X. S2CID 98167823.
- ^ "Organic Chemistry-I" (PDF). Nsdl.niscair.res.in. Archived from the original (PDF) on 29 October 2013. Retrieved 17 February 2014.
- ^ "13. Hydrocarbons | Textbooks". Textbook.s-anand.net. Archived from the original on 6 October 2014. Retrieved 17 February 2014.
- ^ William D. McCain (1990). The properties of petroleum fluids. PennWell. ISBN 978-0-87814-335-1.
- ^ Koch, H.; Haaf, W. (1973). "1-Adamantanecarboxylic Acid". Organic Syntheses; Collected Volumes, vol. 5, p. 20.
- ^ "N-HEXANE". Toxicology data network Hazardous Substances Data Bank. National Library of Medicine. Archived from the original on 4 September 2015.
- ^ Centers for Disease Control and Prevention (CDC) (16 November 2001). "n-Hexane-related peripheral neuropathy among automotive technicians--California, 1999-2000". MMWR. Morbidity and Mortality Weekly Report. 50 (45): 1011–1013. ISSN 0149-2195. PMID 11724159.
- ^ "CDC – NIOSH Pocket Guide to Chemical Hazards – Hexane isomers (excluding n-Hexane)". cdc.gov. Archived from the original on 31 October 2015. Retrieved 3 November 2015.
- ^ a b CDC (28 March 2018). "n-Hexane". Centers for Disease Control and Prevention. Retrieved 3 May 2020.
- ^ "Appendix G: 1989 Air Contaminants Update Project - Exposure Limits NOT in Effect". www.cdc.gov. 20 February 2020. Retrieved 3 May 2020.
- ^ Gad, Shayne C (2005), "Petroleum Hydrocarbons", Encyclopedia of Toxicology, vol. 3 (2nd ed.), Elsevier, pp. 377–379
- ^ a b Clough, Stephen R; Mulholland, Leyna (2005). "Hexane". Encyclopedia of Toxicology. Vol. 2 (2nd ed.). Elsevier. pp. 522–525.
- ^ Rizzuto, N; De Grandis, D; Di Trapani, G; Pasinato, E (1980). "N-hexane polyneuropathy. An occupational disease of shoemakers". European Neurology. 19 (5): 308–15. doi:10.1159/000115166. PMID 6249607.
- ^ n-Hexane, Environmental Health Criteria, World Health Organization, 1991, archived from the original on 19 March 2014
- ^ Liu, C. H.; Huang, C. Y.; Huang, C. C. (2012). "Occupational Neurotoxic Diseases in Taiwan". Safety and Health at Work. 3 (4): 257–67. doi:10.5491/SHAW.2012.3.4.257. PMC 3521924. PMID 23251841.
- ^ "Workers poisoned while making iPhones – ABC News (Australian Broadcasting Corporation)". Australian Broadcasting Corporation. 26 October 2010. Archived from the original on 8 April 2011. Retrieved 17 March 2015.
- ^ David Barboza (22 February 2011). "Workers Sickened at Apple Supplier in China". The New York Times. Archived from the original on 7 April 2015. Retrieved 17 March 2015.
- ^ Filser JG, Csanády GA, Dietz W, Kessler W, Kreuzer PE, Richter M, Störmer A (1996). "Comparative Estimation of the Neurotoxic Risks of N-Hexane and N-Heptane in Rats and Humans Based on the Formation of the Metabolites 2,5-Hexanedione and 2,5-Heptanedione". Biological Reactive Intermediates V. Advances in Experimental Medicine and Biology. Vol. 387. pp. 411–427. doi:10.1007/978-1-4757-9480-9_50. ISBN 978-1-4757-9482-3. PMID 8794236.
External links[edit]
- International Chemical Safety Card 1262 (2-methylpentane)
- Material Safety Data Sheet for Hexane
- National Pollutant Inventory – n-hexane fact sheet
- Phytochemica l database entry
- Center for Disease Control and Prevention
- Warning from National Safety Council "COMMON CHEMICAL AFFECTS AUTO MECHANICS"
- Australian National Pollutant Inventory (NPI) page
- "EPA does not consider n-hexane classifiable as a human carcinogen." Federal Register / Vol. 66, No. 71 / Thursday, 12 April 2001 / Rules and Regulations

