Roussin's red salt

| |
| |
| Names | |
|---|---|
| IUPAC name
potassium tetranitrosyl-di-μ-sulfidodiiron(Fe–Fe)(2–)
| |
| Other names
Ferrate(2-), tetranitrosyldi-mu-thioxodi-, (Fe-Fe), dipotassium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Fe2N4K2O4S2 | |
| Molar mass | 374.04 g/mol |
| Appearance | Dark red crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
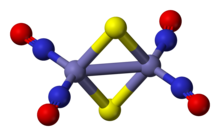
Roussin's red salt is the inorganic compound with the formula K2[Fe2S2(NO)4]. This metal nitrosyl was first described by Zacharie Roussin in 1858, making it one of the first synthetic iron-sulfur clusters.[1][2]
Structure and bonding
[edit]Roussin's red salt anion is an edge-shared bitetrahedron, wherein a pair Fe(NO)2 units are bridged by a pair of sulfide ligands. The Fe-NO bonds are linear indicating NO is acting as a three electron donor.[3] The diamagnetic compound obeys the 18-electron rule. The dark red colour of the complex is attributed to a number of charge-transfer interactions between the iron core and nitrosyl ligands.[4]
Synthesis
[edit]
The French chemist Z. Roussin[5] first prepared this salt while investigating reactions between nitroprusside ion ([Fe(CN)5NO]2−) and sulfur.[6] The salt can be prepared by the reaction of sulfide salts with iron nitrosyl halides:[7]
- Fe2I2(NO)4 + 2Li2S → Li2Fe2S2(NO)4 + 2LiI
Another way to obtain Roussin's red salt is to alkalize a solution of the related compound Roussin's black salt, K[Fe4S3(NO)7], using a suitable base:
- 2K[Fe4S3(NO)7] + 4KOH → 3K2[Fe2S2(NO)4] + 2Fe(OH)2 + 2NO
Another obtaining procedure consists of reacting sodium (or potassium) nitrite, ascorbic acid, ferrous sulfate, sodium sulfide and sodium (or potassium) hydroxide in this order. Ascorbic acid works both as an acidulant and as a reducing agent, converting nitrite into nitrous acid and then reducing it to nitrogen monoxide, which will then bind to iron ions forming nitrosyl complexes, which will react with sulfide in a basic medium giving Roussin's red salt.[8]
- FeSO4 + 4HNO2 + 3C6H8O6 + 2Na2S + 2NaOH → Na2[Fe2S2(NO)4] + 3C6H6O6 + 2Na2SO4 + 6H2O
To obtain the "esters", the salt is alkylated:
- Li2Fe2S2(NO)4 + 2 RX → Fe2(SR)2(NO)4 + 2 LiX
Esters can also be easily be prepared from the reaction of Fe2I2(NO)4 with the thiol or thiosulfate.
Occurrence and potential applications
[edit]It is found in nature as its "esters" with the formula Fe2(SR)2(NO)4, where "R" is any alkyl group.[3] In addition Roussin's red salt is discussed in the fields of microbiology and food science due to its mutagenic properties.[9]
The ester derivative are being investigated as nitric oxide donors in biology and medicine, due to the relatively low toxicity and good stability of Roussin's red salt.[10]Photodissociation of the compound induces the release of NO, thereby sensitizing target cells to exposure to radiation.[9]
See also
[edit]References
[edit]- ^ Butler, Anthony R. (July 1982). "The chemist Z. Roussin (1827-94)". Journal of Chemical Education. 59 (7): 549. Bibcode:1982JChEd..59..549B. doi:10.1021/ed059p549.
- ^ Roussin, M. L. (1858). "Recherches sur les nitrosulfures doubles de fer (nouvelle classe de sels)". Ann. Chim. Phys. 52: 285–303.
- ^ a b Thomas, J. T.; Robertson, J. H.; Cox, E. G. (1 September 1958). "The crystal structure of Roussin's red ethyl ester". Acta Crystallographica. 11 (9): 599–604. doi:10.1107/S0365110X58001602.
- ^ Jaworska, Maria; Stasicka, Zofia (2005). "Structure and UV-Vis spectroscopy of the iron-sulfur dinuclear nitrosyl complexes [Fe2S2(NO)4]2− and [Fe2(SR)2(NO)4]". New Journal of Chemistry. 29 (4): 604. doi:10.1039/B409519G.
- ^ Butler, Anthony R. (July 1982). "The chemist Z. Roussin (1827-94)". Journal of Chemical Education. 59 (7): 549. Bibcode:1982JChEd..59..549B. doi:10.1021/ed059p549.
- ^ Hans Reihlen, Adolf v. Friedolsheim (1927). "Über komplexe Stickoxydverbindungen und das sogenannte einwertige Eisen". Justus Liebigs Annalen der Chemie. 457: 71–82. doi:10.1002/jlac.19274570103.
- ^ TB Rauchfuss; TD Weatherill (1982). "Roussin's Red Salt revisited: reactivity of Fe2 (μ-E) 2 (NO) 42-(E= S, Se, Te) and related". Inorganic Chemistry. 21 (2): 827–830. doi:10.1021/ic00132a071.
- ^ lack sources
- ^ a b Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Yoon, H.; Park, S.; Lim, M. (2020). "Photorelease Dynamics of Nitric Oxide from Cysteine-Bound Roussin's Red Ester". The Journal of Physical Chemistry Letters. 11 (9): 3198–3202. doi:10.1021/acs.jpclett.0c00739. PMID 32250631. S2CID 215408785.
