Phagocyte: Difference between revisions
removed merge template |
Cyclonenim (talk | contribs) m formatting: 11x mdash, 2x heading-style, whitespace (using Advisor.js) |
||
| Line 14: | Line 14: | ||
| issn = |
| issn = |
||
| accessdate = 2008-11-01 |
| accessdate = 2008-11-01 |
||
}}</ref> Phagocytes ingest pathogenic and infectious agents in the body.<ref>{{cite journal|title=Antimicrobial functions of mononuclear phagocytes|journal=Journal of Immunological Methods|date=1994-09-14|first=JA|last=Langermans|coauthors=WL Hazenbos, R van Furth|volume=174|issue=1-2|pages=185-94|pmid=8083520 |url=http://www.ncbi.nlm.nih.gov/pubmed/8083520|format=|accessdate=2008-11-13 }}</ref> Like all cells that are involved in the [[immune system]] they originate in the [[bone marrow]]. Phagocytes derive from a group of [[stem cells]] in the bone marrow called [[myeloid progenitor cells]]. Phagocytes are the basis of defense in the [[innate immune system]]: these cells ingest pathogens and often take part in [[antigen presentation]].<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
}}</ref> Phagocytes ingest pathogenic and infectious agents in the body.<ref>{{cite journal|title=Antimicrobial functions of mononuclear phagocytes|journal=Journal of Immunological Methods|date=1994-09-14|first=JA|last=Langermans|coauthors=WL Hazenbos, R van Furth|volume=174|issue=1-2|pages=185-94|pmid=8083520 |url=http://www.ncbi.nlm.nih.gov/pubmed/8083520|format=|accessdate=2008-11-13 }}</ref> Like all cells that are involved in the [[immune system]] they originate in the [[bone marrow]]. Phagocytes derive from a group of [[stem cells]] in the bone marrow called [[myeloid progenitor cells]]. Phagocytes are the basis of defense in the [[innate immune system]]: these cells ingest pathogens and often take part in [[antigen presentation]].<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> Their name comes from the Greek word phagein (meaning to eat or devour) and from the Greek word kutos (meaning hollow vessel).<ref> {{cite web|url=http://dictionary.reference.com/browse/phago- |title=phago- |accessdate=2008-11-12 |work=Dictionary.com }}</ref><ref> {{cite web|url=http://dictionary.reference.com/browse/-cyte |title=-cyte |accessdate=2008-11-13 |work=Dictionary.com }}</ref> The types of phagocytes include [[neutrophils]], [[macrophages]], and [[monocytes]].<ref> {{cite web|url=http://www.dent.ucla.edu/pic/members/neutrophils/neutrophils.html |title=Phagocytes-Neutrophils |accessdate=2008-11-13 |last=Miyasaki |first=Ken }}</ref> [[Dendritic cells]] also participate in [[phagocytosis]] and presentation of antigens to other cells that are important in the immune response.<ref name=antigen> {{cite journal|title=Antigen presentation and T cell stimulation by dendritic cells.|journal=Annual Review of Immunology|date=2002|first=P|last=Guermonprez|coauthors=J Valladeau, L Zitvogel, C Thery, S Amigorena|volume=20|issue=|pages=621-67|pmid=11861614 |url=http://www.ncbi.nlm.nih.gov/pubmed/11861614|format=|accessdate=2008-11-12 }}</ref> |
||
==History== |
==History== |
||
| Line 22: | Line 22: | ||
==Phagocytosis== |
==Phagocytosis== |
||
{{main|Phagocytosis}} |
{{main|Phagocytosis}} |
||
Phagocytosis is the process of taking in foreign material. <ref> {{cite journal|title=Phagocytosis and the actin cytoskeleton.|journal=Journal of Cell Science|date=2001|first=RC|last=May|coauthors=LM Machesky|volume=114|issue=6|pages=119-33|pmid=11228151 |url=http://www.ncbi.nlm.nih.gov/pubmed/11228151|format=|accessdate=2008-11-13 }}</ref> It is one of the of the [[endocytosis|endocytic]] processes. <ref name=relation> {{cite journal|title=Relationship between ultrastructure and specific functions of macrophages.|journal=Comparative Immunology, Microbiology and Infectious Diseases|date=1985|first=A|last=Ryter|coauthors=|volume=8|issue=2|pages=119-33|pmid=3910340 |url=http://www.ncbi.nlm.nih.gov/pubmed/3910340|format=|accessdate=2008-11-13 }}</ref> Phagocytosis occurs after the bacterium is bound to one of the receptors. In this process the phagocyte stretches its [[pseudopodium]] around the bacterium and engulfs it. The bacterium is then trapped in a [[phagosome]]. The phagosome then combines with either a [[lysosome]] or a [[granule]] (from a neutrophil). The contents of the granule or lysosome are then released into the phagosome—the combination of a phagosome and a lysosome (or granule) produces a [[phagolysosome]]. <ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
Phagocytosis is the process of taking in foreign material. <ref> {{cite journal|title=Phagocytosis and the actin cytoskeleton.|journal=Journal of Cell Science|date=2001|first=RC|last=May|coauthors=LM Machesky|volume=114|issue=6|pages=119-33|pmid=11228151 |url=http://www.ncbi.nlm.nih.gov/pubmed/11228151|format=|accessdate=2008-11-13 }}</ref> It is one of the of the [[endocytosis|endocytic]] processes. <ref name=relation> {{cite journal|title=Relationship between ultrastructure and specific functions of macrophages.|journal=Comparative Immunology, Microbiology and Infectious Diseases|date=1985|first=A|last=Ryter|coauthors=|volume=8|issue=2|pages=119-33|pmid=3910340 |url=http://www.ncbi.nlm.nih.gov/pubmed/3910340|format=|accessdate=2008-11-13 }}</ref> Phagocytosis occurs after the bacterium is bound to one of the receptors. In this process the phagocyte stretches its [[pseudopodium]] around the bacterium and engulfs it. The bacterium is then trapped in a [[phagosome]]. The phagosome then combines with either a [[lysosome]] or a [[granule]] (from a neutrophil). The contents of the granule or lysosome are then released into the phagosome—the combination of a phagosome and a lysosome (or granule) produces a [[phagolysosome]]. <ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> |
||
===Initiation of phagocytosis=== |
===Initiation of phagocytosis=== |
||
A phagocyte has receptors on its surface that are used to bind infectious agents to itself.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
A phagocyte has receptors on its surface that are used to bind infectious agents to itself.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> These receptors increase the ability of a phagocyte to phagocytize foreign material.<ref name=relation> {{cite journal|title=Relationship between ultrastructure and specific functions of macrophages.|journal=Comparative Immunology, Microbiology and Infectious Diseases|date=1985|first=A|last=Ryter|coauthors=|volume=8|issue=2|pages=119-33|pmid=3910340 |url=http://www.ncbi.nlm.nih.gov/pubmed/3910340|format=|accessdate=2008-11-13 }}</ref> These receptors include [[Fc receptors]], [[complement]] receptors, scavenger receptors, and [[toll-like receptors]]. Fc receptors increase the phagoctyosis of bacteria that have been coated with [[IgG]] [[antibodies]]. When bacteria coated with IgG antibodies are bound to the Fc receptors, this increases the metabolic activity of phagocytes used in intracellular killing. Complement receptors bind bacteria coated with complement [[C3b]]. Binding to the complement receptors increases phagocytosis and intracellular killing. Scavenger receptors bind to a large range of molecules on the surface of bacterial cells, and this increases the phagocytosis of bacteria. Toll-like receptors bind to more specific molecules. Binding to toll-like receptors increases phagocytosis and causes the phagocyte to release a group of cytokines related to [[inflammation]].<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> |
||
==Migration== |
==Migration== |
||
| Line 31: | Line 31: | ||
===Initial signaling=== |
===Initial signaling=== |
||
When infection occurs, a signal (SOS signals) is given off to attract monocyte (macrophage <ref name=mono> {{cite web|url=http://www.healthsystem.virginia.edu/internet/hematology/HessEDD/BenignHematologicDisorders/normal-hematopoietic-cells/Monocyte.cfm |title=Monocyte |accessdate=2008-11-12 |last=Hess |first=Charles E. }}</ref> and dendritic cell precursors <ref name=dendrite> {{cite web|url=http://www.healthsystem.virginia.edu/internet/hematology/HessEDD/BenignHematologicDisorders/normal-hematopoietic-cells/Dendritic-cell.cfm |title=Dendritic Cell |accessdate=2008-11-12 |last=Hess |first=Charles E. }}</ref>) and neutrophils. Chemical signals may include N-formyl-methionine peptides that originate in invading [[bacteria]], clotting system [[peptides]], [[complement]] products, and [[cytokines]] that have been given off by macrophages located in the tissue near the infection site.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
When infection occurs, a signal (SOS signals) is given off to attract monocyte (macrophage <ref name=mono> {{cite web|url=http://www.healthsystem.virginia.edu/internet/hematology/HessEDD/BenignHematologicDisorders/normal-hematopoietic-cells/Monocyte.cfm |title=Monocyte |accessdate=2008-11-12 |last=Hess |first=Charles E. }}</ref> and dendritic cell precursors <ref name=dendrite> {{cite web|url=http://www.healthsystem.virginia.edu/internet/hematology/HessEDD/BenignHematologicDisorders/normal-hematopoietic-cells/Dendritic-cell.cfm |title=Dendritic Cell |accessdate=2008-11-12 |last=Hess |first=Charles E. }}</ref>) and neutrophils. Chemical signals may include N-formyl-methionine peptides that originate in invading [[bacteria]], clotting system [[peptides]], [[complement]] products, and [[cytokines]] that have been given off by macrophages located in the tissue near the infection site.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> Another group of chemical attractants are [[chemokines]] (a type of cytokine) that are released by phagocytes near the infection. Like the other attractants, chemokines serve as recruiting agent for neutrophils and monocytes. For example, interleukin-8 attracts neutrophils from the blood stream into surrounding tissues, and macrophage chemoattractant protein-1 causes monocytes to leave the blood stream and enter tissues near the infection where the monocytes then develop into tissue macrophages..<ref name=money>{{cite book | last = Janeway | first = Charles A. | authorlink = | coauthors = Kenneth M. Murphy, Paul Travers, Mark Walport | title = Induced innate responses to infection. | publisher = | date = | location = | pages = | url = http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=10 | doi = | id = | isbn = 978-0-8153-4123-9 }}</ref> |
||
===Endothelial and epithelial migration=== |
===Endothelial and epithelial migration=== |
||
Signaling then promotes the phagocytes to attach to cell adhesion molecules. [[Selectins]] are the first group of endothelial adhesion molecules. Selectins—cytokines from macrophages are responsible for the release of granules found in endothelial cells that contain P-selectins— are found on the membrane of the endothelial cell and bond with certain carbohydrate groups, like the oligosaccharides on the surface of the monocytes and neutrophils. Intracellular adhesion molecules (or ICAMs) are responsible for producing a tighter attachment to the phagocyte. These molecules form bonds with the integral proteins on the surface of the circulating monocytes and neutrophils. ICAM-1 promotes strong endothelial and phagocytic bonds on the surface of irritated [[endothelial cells]]. Chemokines also help to create a better connection by changing the shape of molecules such as leukocyte functional antigen-1 (LFA-1) found on traveling monocytes and neutrophils. While ICAM-1 binds to LFA-1 on both neutrophils and monocytes (after exposure to the macrophage cytokine TNF-a), ICAM-2 is used to help only monocytes get into the infected tissue.<ref name=money>{{cite book | last = Janeway | first = Charles A. | authorlink = | coauthors = Kenneth M. Murphy, Paul Travers, Mark Walport | title = Induced innate responses to infection. | publisher = | date = | location = | pages = | url = http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=10 | doi = | id = | isbn = 978-0-8153-4123-9 }}</ref> |
Signaling then promotes the phagocytes to attach to cell adhesion molecules. [[Selectins]] are the first group of endothelial adhesion molecules. Selectins—cytokines from macrophages are responsible for the release of granules found in endothelial cells that contain P-selectins— are found on the membrane of the endothelial cell and bond with certain carbohydrate groups, like the oligosaccharides on the surface of the monocytes and neutrophils. Intracellular adhesion molecules (or ICAMs) are responsible for producing a tighter attachment to the phagocyte. These molecules form bonds with the integral proteins on the surface of the circulating monocytes and neutrophils. ICAM-1 promotes strong endothelial and phagocytic bonds on the surface of irritated [[endothelial cells]]. Chemokines also help to create a better connection by changing the shape of molecules such as leukocyte functional antigen-1 (LFA-1) found on traveling monocytes and neutrophils. While ICAM-1 binds to LFA-1 on both neutrophils and monocytes (after exposure to the macrophage cytokine TNF-a), ICAM-2 is used to help only monocytes get into the infected tissue.<ref name=money>{{cite book | last = Janeway | first = Charles A. | authorlink = | coauthors = Kenneth M. Murphy, Paul Travers, Mark Walport | title = Induced innate responses to infection. | publisher = | date = | location = | pages = | url = http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=10 | doi = | id = | isbn = 978-0-8153-4123-9 }}</ref> |
||
Other signals from the infection site called vasodilators enable the phagocytes to cross through the spaces of endothelial cells by loosening the junctions connecting them (a process called diapedesis). Once the phagocytes are in the tissue in which the infection is occurring, [[chemotaxis]] allows the phagocytes to find the exact area. SOS signals may also enhance a phagocyte’s ability to ingest and kill organisms through the respective processes of phagocytosis and intracellular killing.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
Other signals from the infection site called vasodilators enable the phagocytes to cross through the spaces of endothelial cells by loosening the junctions connecting them (a process called diapedesis). Once the phagocytes are in the tissue in which the infection is occurring, [[chemotaxis]] allows the phagocytes to find the exact area. SOS signals may also enhance a phagocyte’s ability to ingest and kill organisms through the respective processes of phagocytosis and intracellular killing.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> |
||
Neutrophils also travel across [[epithelial]]-lined [[organs]] to sites of infection. This involves a series of interactions that have not yet been fully studied. Several protein interactions that have been identified are those between leukocyte CD11b (and CD18) with fucosylated glycoproteins that have been expressed by signaling. Following this reaction is a binding of the leukocyte proteins and desmosomal-associated JAM-C. Two other binding proteins have also been studied: junctional adhesion molecule-like protein (from the neutrophil) and epithelial coxsackie and adenovirus receptor.<ref> {{cite journal|title=Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils.|journal=Molecular Biology of the Cell|date=2005|first=K|last=Zen|coauthors=Y Liu, IC McCall, T Wu, W Lee, BA Babbin, A Nusrat, CA Parkos|volume=16|issue=6|pages=2694-703|pmid=15800062 |url=http://www.ncbi.nlm.nih.gov/pubmed/15800062|format=|accessdate=2008-11-13 }}</ref> Although neutrophil migration across epithelial-lined organs is an important component of fighting [[infection]], the migration itself can result in disease-like symptoms.<ref> {{cite journal|title=Leukocyte-epithelial interactions.|journal=Current Opinion in Cell Biology|date=2003|first=K|last=Zen|coauthors=CA Parkos|volume=15|issue=5|pages=557-64|pmid=14519390 |url=http://www.ncbi.nlm.nih.gov/pubmed/14519390|format=|accessdate=2008-11-12 }}</ref> |
Neutrophils also travel across [[epithelial]]-lined [[organs]] to sites of infection. This involves a series of interactions that have not yet been fully studied. Several protein interactions that have been identified are those between leukocyte CD11b (and CD18) with fucosylated glycoproteins that have been expressed by signaling. Following this reaction is a binding of the leukocyte proteins and desmosomal-associated JAM-C. Two other binding proteins have also been studied: junctional adhesion molecule-like protein (from the neutrophil) and epithelial coxsackie and adenovirus receptor.<ref> {{cite journal|title=Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils.|journal=Molecular Biology of the Cell|date=2005|first=K|last=Zen|coauthors=Y Liu, IC McCall, T Wu, W Lee, BA Babbin, A Nusrat, CA Parkos|volume=16|issue=6|pages=2694-703|pmid=15800062 |url=http://www.ncbi.nlm.nih.gov/pubmed/15800062|format=|accessdate=2008-11-13 }}</ref> Although neutrophil migration across epithelial-lined organs is an important component of fighting [[infection]], the migration itself can result in disease-like symptoms.<ref> {{cite journal|title=Leukocyte-epithelial interactions.|journal=Current Opinion in Cell Biology|date=2003|first=K|last=Zen|coauthors=CA Parkos|volume=15|issue=5|pages=557-64|pmid=14519390 |url=http://www.ncbi.nlm.nih.gov/pubmed/14519390|format=|accessdate=2008-11-12 }}</ref> |
||
| Line 43: | Line 43: | ||
===Oxygen-dependent intracellular killing=== |
===Oxygen-dependent intracellular killing=== |
||
When a phagocyte phagocytizes bacteria (or any material) its oxygen consumption increases. The increase in oxygen consumption is called a [[respiratory burst]]. A respiratory burst results in the production of anti-microbial reactive oxygen-containing molecules.<ref> {{cite journal|title=Respiratory burst in human neutrophils.|journal=Journal of Immunological Methods|date=1999-12-17|first=C|last=Dahlgren|coauthors=A Karlsson|volume=232|issue=1-2|pages=3-14|pmid=10618505 |url=http://www.ncbi.nlm.nih.gov/pubmed/10618505|format=|accessdate=2008-11-13 }}</ref> Killing invading microbes by using the reactive oxygen-containing molecules is referred to as oxygen-dependent intracellular killing. The oxygen compounds are toxic to both the invader and the cell itself, so the phagocyte uses a series of [[detoxification]] reactions to protect itself by breaking down the substances. There are two types of oxygen-dependent intracellular killing methods. The first type is oxygen-dependent myeloperoxidase-independent intracellular killing. When [[glucose]] is used during phagocytosis, it is converted into [[NADPH]]. Then [[NADPH oxidase]] is activated, this enzyme’s role is to oxidize NADPH. The oxidation of NADPH creates superoxide anion.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
When a phagocyte phagocytizes bacteria (or any material) its oxygen consumption increases. The increase in oxygen consumption is called a [[respiratory burst]]. A respiratory burst results in the production of anti-microbial reactive oxygen-containing molecules.<ref> {{cite journal|title=Respiratory burst in human neutrophils.|journal=Journal of Immunological Methods|date=1999-12-17|first=C|last=Dahlgren|coauthors=A Karlsson|volume=232|issue=1-2|pages=3-14|pmid=10618505 |url=http://www.ncbi.nlm.nih.gov/pubmed/10618505|format=|accessdate=2008-11-13 }}</ref> Killing invading microbes by using the reactive oxygen-containing molecules is referred to as oxygen-dependent intracellular killing. The oxygen compounds are toxic to both the invader and the cell itself, so the phagocyte uses a series of [[detoxification]] reactions to protect itself by breaking down the substances. There are two types of oxygen-dependent intracellular killing methods. The first type is oxygen-dependent myeloperoxidase-independent intracellular killing. When [[glucose]] is used during phagocytosis, it is converted into [[NADPH]]. Then [[NADPH oxidase]] is activated, this enzyme’s role is to oxidize NADPH. The oxidation of NADPH creates superoxide anion.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> Superoxide anion is an important microbicidal substance in phagocytes.<ref> {{cite journal|title=NADPH oxidase.|journal=The international journal of biochemistry and cell biology.|date=1996|first=KP|last=Shatwell|coauthors=AW Segal|volume=28|issue=11|pages=1191-5|id=PMID 9022278 |url=http://www.ncbi.nlm.nih.gov/sites/entrez|format=|accessdate=2008-12-30 }}</ref> The superoxide anion is then converted to [[hydrogen peroxide]] and [[singlet oxygen]] with the help of the enzyme [[superoxide dismutase]]. In addition to these compounds, superoxide anion reacts with hydrogen peroxide to produce [[hydroxyl radicals]]. All of these products are used to kill the invading microbe.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> The next type, oxygen-dependent myeloperoxidase-dependent intracellular killing, occurs in neutrophils and monocytes because it involves the use of [[myeloperoxidase]] from granules.<ref> {{cite journal|title=Myeloperoxidase.|journal=Proceedings of the Association of American Physicians|date=1999|first=SJ|last=Klebenoff|coauthors=|volume=111|issue=5|pages=383-9|id=PMID 10519157 |url=http://www.ncbi.nlm.nih.gov/sites/entrez|format=|accessdate=2008-12-30 }}</ref> When granules fuse with a phagosome myeloperoxidase is released into the phagolysosome—this enzyme uses hydrogen peroxide and [[halide]] ions (primarily chloride ions) to create [[hypochlorite]]. Hypochlorite is an extremely toxic substance that can be broken down by itself into singlet oxygen. Both the hypochlorite and the singlet oxygen are used to kill microbes in the phagolysosome.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> |
||
===Oxygen-independent intracellular killing=== |
===Oxygen-independent intracellular killing=== |
||
Another way that phagocytes kill microbes is by oxygen-independent methods. However, these methods are not as effective as the oxygen-dependent methods. There are four main types of oxygen-independent methods. The first type is when cationic proteins are used; when the phagosome becomes a phagolysosome these proteins are released and are used to damage the bacterium’s [[cell membrane|membrane]]. The second type is when lysozymes are used; these enzymes are used to break down the bacterial [[cell wall]]. The third type makes use of lactoferrins; they are used to take away iron from the bacterium. The fourth type uses proteolytic and [[hydrolytic enzymes]]; these enzymes are used to digest the proteins of killed bacteria.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
Another way that phagocytes kill microbes is by oxygen-independent methods. However, these methods are not as effective as the oxygen-dependent methods. There are four main types of oxygen-independent methods. The first type is when cationic proteins are used; when the phagosome becomes a phagolysosome these proteins are released and are used to damage the bacterium’s [[cell membrane|membrane]]. The second type is when lysozymes are used; these enzymes are used to break down the bacterial [[cell wall]]. The third type makes use of lactoferrins; they are used to take away iron from the bacterium. The fourth type uses proteolytic and [[hydrolytic enzymes]]; these enzymes are used to digest the proteins of killed bacteria.<ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> |
||
==Extracellular killing== |
==Extracellular killing== |
||
In macrophages, [[IFN-gamma]] stimulates the production of [[nitric oxide]] by increasing the use of inducible nitric oxide synthase ([[iNOS]]). [[TNF-alpha]] is also used in this process to promote anti-microbial iNOS methods. <ref name=popcornchicken>{{cite book | last = Masek | first = Katherine S. | authorlink = | coauthors = Christopher A. Hunter | title = Eurekah Bioscience Collection: Macrophage Effector Functions | publisher = Landes Bioscience | date = 2007 | location = | pages = | url = http://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=macrophage,functions,effector&rid=eurekah.section.68254 | doi = | id = | isbn = }}</ref> Nitric oxide is then released from the macrophage; and, because of its toxicity, kills invading microbes near the macrophage. <ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology |
In macrophages, [[IFN-gamma]] stimulates the production of [[nitric oxide]] by increasing the use of inducible nitric oxide synthase ([[iNOS]]). [[TNF-alpha]] is also used in this process to promote anti-microbial iNOS methods. <ref name=popcornchicken>{{cite book | last = Masek | first = Katherine S. | authorlink = | coauthors = Christopher A. Hunter | title = Eurekah Bioscience Collection: Macrophage Effector Functions | publisher = Landes Bioscience | date = 2007 | location = | pages = | url = http://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=macrophage,functions,effector&rid=eurekah.section.68254 | doi = | id = | isbn = }}</ref> Nitric oxide is then released from the macrophage; and, because of its toxicity, kills invading microbes near the macrophage. <ref name=USC>{{cite web | last = Mayer | first = Gene |title=Immunology — Chapter One: Innate (non-specific) Immunity | work = Microbiology and Immunology On-Line Textbook | publisher = USC School of Medicine | year = 2006|url=http://pathmicro.med.sc.edu/ghaffar/innate.htm | accessdate = 2008-11-12}}</ref> |
||
==Antigen presentation== |
==Antigen presentation== |
||
| Line 70: | Line 70: | ||
===Neutrophils=== |
===Neutrophils=== |
||
{{main|Neutrophils}} |
{{main|Neutrophils}} |
||
Neutrophils participate in phagocytosis of antibody and complement coated antigens. They can also phagocytize damaged cells or cell parts. Neutrophils have a segmented [[nucleus]]. This means that they have a nucleus that has several sections; each section is connected by [[chromatin]] filaments—neutrophils can have 2-5 segments. Neutrophils do not normally exit the bone marrow until their nucleus has been segmented; but if there is a high need for neutrophils or if there are irregularities in the bone marrow, neutrophil precursors called myelocytes and promyelocytes are released. Neutrophils are also separated between circulating and marginal groups (about 50% of neutrophils are marginated). <ref> {{cite web|url=http://www.healthsystem.virginia.edu/internet/hematology/HessEDD/BenignHematologicDisorders/normal-hematopoietic-cells/segmented-neutrophil.cfm |title=Segmented Neutrophil |accessdate=2008-11-14 |last=Hess |first=Charles |publisher=University of Virginia Health System }}</ref> |
Neutrophils participate in phagocytosis of antibody and complement coated antigens. They can also phagocytize damaged cells or cell parts. Neutrophils have a segmented [[nucleus]]. This means that they have a nucleus that has several sections; each section is connected by [[chromatin]] filaments—neutrophils can have 2-5 segments. Neutrophils do not normally exit the bone marrow until their nucleus has been segmented; but if there is a high need for neutrophils or if there are irregularities in the bone marrow, neutrophil precursors called myelocytes and promyelocytes are released. Neutrophils are also separated between circulating and marginal groups (about 50% of neutrophils are marginated). <ref> {{cite web|url=http://www.healthsystem.virginia.edu/internet/hematology/HessEDD/BenignHematologicDisorders/normal-hematopoietic-cells/segmented-neutrophil.cfm |title=Segmented Neutrophil |accessdate=2008-11-14 |last=Hess |first=Charles |publisher=University of Virginia Health System }}</ref> |
||
Neutrophils can also secrete products that stimulate monocytes and macrophages. Neutrophil secretions increase phagocytosis and the formation of reactive oxygen compounds involved in intracellular killing. <ref> {{cite journal|title=Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages.|journal=Clinical and Experimental Immunology|date=2008|first=O|last=Soehnlein|coauthors=E Kenne, P Rotzius, EE Eriksson, L Lindbom|volume=151|issue=1|pages=139-45|pmid=17991288 |url=http://www.ncbi.nlm.nih.gov/pubmed/17991288|format=|accessdate=2008-11-14 }}</ref> Heparin-binding protein and human neutrophil peptides 1-3 have been found to mediate the response to neutrophil secretions. Secretion from the primary granules of neutrophils stimulated the phagocytosis of IgG-coated bacteria. <ref> {{cite journal|title=Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages.|journal=The Journal of Clinical Investigation|date=2008|first=O|last=Soehnlein|coauthors=E Kenne, P Rotzius, EE Eriksson, L Lindbom, Y Kai-Larsen, R Frithiof, OE Sorensen, K Scharffetter-Kochanek, H Herwald, B Agerberth|volume=118|issue=10|pages=3491-502|pmid=18787642 |url=http://www.ncbi.nlm.nih.gov/pubmed/18787642|format=|accessdate=2008-11-14 }}</ref> |
Neutrophils can also secrete products that stimulate monocytes and macrophages. Neutrophil secretions increase phagocytosis and the formation of reactive oxygen compounds involved in intracellular killing. <ref> {{cite journal|title=Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages.|journal=Clinical and Experimental Immunology|date=2008|first=O|last=Soehnlein|coauthors=E Kenne, P Rotzius, EE Eriksson, L Lindbom|volume=151|issue=1|pages=139-45|pmid=17991288 |url=http://www.ncbi.nlm.nih.gov/pubmed/17991288|format=|accessdate=2008-11-14 }}</ref> Heparin-binding protein and human neutrophil peptides 1-3 have been found to mediate the response to neutrophil secretions. Secretion from the primary granules of neutrophils stimulated the phagocytosis of IgG-coated bacteria. <ref> {{cite journal|title=Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages.|journal=The Journal of Clinical Investigation|date=2008|first=O|last=Soehnlein|coauthors=E Kenne, P Rotzius, EE Eriksson, L Lindbom, Y Kai-Larsen, R Frithiof, OE Sorensen, K Scharffetter-Kochanek, H Herwald, B Agerberth|volume=118|issue=10|pages=3491-502|pmid=18787642 |url=http://www.ncbi.nlm.nih.gov/pubmed/18787642|format=|accessdate=2008-11-14 }}</ref> |
||
| Line 175: | Line 175: | ||
}}</ref> (this also results in the release of the contents in a neutrophil’s granules), and using exotoxins (these toxins can reduce the supply of a phagocyte's [[ATP]], which is needed for phagocytosis). After a bacterium is ingested it may kill the phagocyte by releasing toxins that travel through the phagosome or phagolysosome membrane to target other parts of the cell. <ref name=chicken> {{cite web|url=http://textbookofbacteriology.net/antiphago.html |title=Mechanisms of bacterial pathogenicity: Bacterial defense against phagocytes |accessdate=2008-12-10 |last=Todar |first=Kenneth |publisher=2008 }}</ref> |
}}</ref> (this also results in the release of the contents in a neutrophil’s granules), and using exotoxins (these toxins can reduce the supply of a phagocyte's [[ATP]], which is needed for phagocytosis). After a bacterium is ingested it may kill the phagocyte by releasing toxins that travel through the phagosome or phagolysosome membrane to target other parts of the cell. <ref name=chicken> {{cite web|url=http://textbookofbacteriology.net/antiphago.html |title=Mechanisms of bacterial pathogenicity: Bacterial defense against phagocytes |accessdate=2008-12-10 |last=Todar |first=Kenneth |publisher=2008 }}</ref> |
||
== |
==References== |
||
{{reflist|colwidth=30em}} |
{{reflist|colwidth=30em}} |
||
== |
==External links== |
||
* {{MeshName|Phagocytes}} |
* {{MeshName|Phagocytes}} |
||
Revision as of 23:32, 3 January 2009
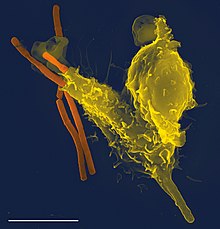
Phagocytes are cells that are found in the blood, bone marrow and other tissues of vertebrates.[1] Phagocytes ingest pathogenic and infectious agents in the body.[2] Like all cells that are involved in the immune system they originate in the bone marrow. Phagocytes derive from a group of stem cells in the bone marrow called myeloid progenitor cells. Phagocytes are the basis of defense in the innate immune system: these cells ingest pathogens and often take part in antigen presentation.[3] Their name comes from the Greek word phagein (meaning to eat or devour) and from the Greek word kutos (meaning hollow vessel).[4][5] The types of phagocytes include neutrophils, macrophages, and monocytes.[6] Dendritic cells also participate in phagocytosis and presentation of antigens to other cells that are important in the immune response.[7]
History
In 1882 Ilya Ilyich Mechnikov studied motile cells in the larvae of starfishes that he believed were important to their immune defenses. To test his idea he inserted small thorns from a tangerine tree into the larvae. He noticed that the motile cells surrounded the thorns. Mechnikov knew that in animals that have a vascular system leukocytes migrate from the blood stream during an infection. He deduced that these leukocytes might migrate from the blood to engulf and digest bacteria. Mechnikov went to Vienna and explained his ideas to a Professor Claus (of Zoology). It was Professor Claus who created the name phagocyte. To advance his hypothesis, Mechnikov studied a fresh-water crustacean called Daphnia. He discovered that fungal spores that attacked the crustacean were destroyed by phagocytes. He later studied the bacterium Bacillus anthracis and found that this organism could also be destroyed by phagocytes.[8] Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine for his discovery of phagocytes and phagocytosis.[9]
Phagocytosis
Phagocytosis is the process of taking in foreign material. [10] It is one of the of the endocytic processes. [11] Phagocytosis occurs after the bacterium is bound to one of the receptors. In this process the phagocyte stretches its pseudopodium around the bacterium and engulfs it. The bacterium is then trapped in a phagosome. The phagosome then combines with either a lysosome or a granule (from a neutrophil). The contents of the granule or lysosome are then released into the phagosome—the combination of a phagosome and a lysosome (or granule) produces a phagolysosome. [3]
Initiation of phagocytosis
A phagocyte has receptors on its surface that are used to bind infectious agents to itself.[3] These receptors increase the ability of a phagocyte to phagocytize foreign material.[11] These receptors include Fc receptors, complement receptors, scavenger receptors, and toll-like receptors. Fc receptors increase the phagoctyosis of bacteria that have been coated with IgG antibodies. When bacteria coated with IgG antibodies are bound to the Fc receptors, this increases the metabolic activity of phagocytes used in intracellular killing. Complement receptors bind bacteria coated with complement C3b. Binding to the complement receptors increases phagocytosis and intracellular killing. Scavenger receptors bind to a large range of molecules on the surface of bacterial cells, and this increases the phagocytosis of bacteria. Toll-like receptors bind to more specific molecules. Binding to toll-like receptors increases phagocytosis and causes the phagocyte to release a group of cytokines related to inflammation.[3]
Migration
Initial signaling
When infection occurs, a signal (SOS signals) is given off to attract monocyte (macrophage [12] and dendritic cell precursors [13]) and neutrophils. Chemical signals may include N-formyl-methionine peptides that originate in invading bacteria, clotting system peptides, complement products, and cytokines that have been given off by macrophages located in the tissue near the infection site.[3] Another group of chemical attractants are chemokines (a type of cytokine) that are released by phagocytes near the infection. Like the other attractants, chemokines serve as recruiting agent for neutrophils and monocytes. For example, interleukin-8 attracts neutrophils from the blood stream into surrounding tissues, and macrophage chemoattractant protein-1 causes monocytes to leave the blood stream and enter tissues near the infection where the monocytes then develop into tissue macrophages..[14]
Endothelial and epithelial migration
Signaling then promotes the phagocytes to attach to cell adhesion molecules. Selectins are the first group of endothelial adhesion molecules. Selectins—cytokines from macrophages are responsible for the release of granules found in endothelial cells that contain P-selectins— are found on the membrane of the endothelial cell and bond with certain carbohydrate groups, like the oligosaccharides on the surface of the monocytes and neutrophils. Intracellular adhesion molecules (or ICAMs) are responsible for producing a tighter attachment to the phagocyte. These molecules form bonds with the integral proteins on the surface of the circulating monocytes and neutrophils. ICAM-1 promotes strong endothelial and phagocytic bonds on the surface of irritated endothelial cells. Chemokines also help to create a better connection by changing the shape of molecules such as leukocyte functional antigen-1 (LFA-1) found on traveling monocytes and neutrophils. While ICAM-1 binds to LFA-1 on both neutrophils and monocytes (after exposure to the macrophage cytokine TNF-a), ICAM-2 is used to help only monocytes get into the infected tissue.[14] Other signals from the infection site called vasodilators enable the phagocytes to cross through the spaces of endothelial cells by loosening the junctions connecting them (a process called diapedesis). Once the phagocytes are in the tissue in which the infection is occurring, chemotaxis allows the phagocytes to find the exact area. SOS signals may also enhance a phagocyte’s ability to ingest and kill organisms through the respective processes of phagocytosis and intracellular killing.[3]
Neutrophils also travel across epithelial-lined organs to sites of infection. This involves a series of interactions that have not yet been fully studied. Several protein interactions that have been identified are those between leukocyte CD11b (and CD18) with fucosylated glycoproteins that have been expressed by signaling. Following this reaction is a binding of the leukocyte proteins and desmosomal-associated JAM-C. Two other binding proteins have also been studied: junctional adhesion molecule-like protein (from the neutrophil) and epithelial coxsackie and adenovirus receptor.[15] Although neutrophil migration across epithelial-lined organs is an important component of fighting infection, the migration itself can result in disease-like symptoms.[16]
Intracellular killing
Oxygen-dependent intracellular killing
When a phagocyte phagocytizes bacteria (or any material) its oxygen consumption increases. The increase in oxygen consumption is called a respiratory burst. A respiratory burst results in the production of anti-microbial reactive oxygen-containing molecules.[17] Killing invading microbes by using the reactive oxygen-containing molecules is referred to as oxygen-dependent intracellular killing. The oxygen compounds are toxic to both the invader and the cell itself, so the phagocyte uses a series of detoxification reactions to protect itself by breaking down the substances. There are two types of oxygen-dependent intracellular killing methods. The first type is oxygen-dependent myeloperoxidase-independent intracellular killing. When glucose is used during phagocytosis, it is converted into NADPH. Then NADPH oxidase is activated, this enzyme’s role is to oxidize NADPH. The oxidation of NADPH creates superoxide anion.[3] Superoxide anion is an important microbicidal substance in phagocytes.[18] The superoxide anion is then converted to hydrogen peroxide and singlet oxygen with the help of the enzyme superoxide dismutase. In addition to these compounds, superoxide anion reacts with hydrogen peroxide to produce hydroxyl radicals. All of these products are used to kill the invading microbe.[3] The next type, oxygen-dependent myeloperoxidase-dependent intracellular killing, occurs in neutrophils and monocytes because it involves the use of myeloperoxidase from granules.[19] When granules fuse with a phagosome myeloperoxidase is released into the phagolysosome—this enzyme uses hydrogen peroxide and halide ions (primarily chloride ions) to create hypochlorite. Hypochlorite is an extremely toxic substance that can be broken down by itself into singlet oxygen. Both the hypochlorite and the singlet oxygen are used to kill microbes in the phagolysosome.[3]
Oxygen-independent intracellular killing
Another way that phagocytes kill microbes is by oxygen-independent methods. However, these methods are not as effective as the oxygen-dependent methods. There are four main types of oxygen-independent methods. The first type is when cationic proteins are used; when the phagosome becomes a phagolysosome these proteins are released and are used to damage the bacterium’s membrane. The second type is when lysozymes are used; these enzymes are used to break down the bacterial cell wall. The third type makes use of lactoferrins; they are used to take away iron from the bacterium. The fourth type uses proteolytic and hydrolytic enzymes; these enzymes are used to digest the proteins of killed bacteria.[3]
Extracellular killing
In macrophages, IFN-gamma stimulates the production of nitric oxide by increasing the use of inducible nitric oxide synthase (iNOS). TNF-alpha is also used in this process to promote anti-microbial iNOS methods. [20] Nitric oxide is then released from the macrophage; and, because of its toxicity, kills invading microbes near the macrophage. [3]
Antigen presentation
There are two “professional” antigen presenting cells. They are macrophages and dendritic cells. [21] After phagocytosis, these cells derive antigens from either the pathogen itself or from its products. Protein antigens are turned into peptides inside of the dendritic cells and macrophages; then the peptides are carried to the surface by linking to major histocompatibility complex (MHC) glycoproteins. There are two different classes of MHC molecules that carry peptides originating from different places inside the cell: MHC class I and MHC class II. MHC class I molecules carry peptides from the cytosol to the surface of the cell where CD8 T cells recognize them. MHC class II molecules transport peptides from vesicles to the surface of the cell where they are recognized by CD4 T cells. MHC molecules are polygenic (meaning that the cell possesses several genes that code for each class of MHC molecules) and polymorphic (meaning that the genes have many variations capable of producing different molecules). Because of the MHC molecules’ combination of being both polygenic and polymorphic, it covers a great range of peptides that can be carried to the surface of a cell to be recognized by T cells. [22]
Cell types
Monocytes
Most mature monocytes are slightly larger than neutrophils. Monocytes also have granules. Monocytes phagocytize foreign or dangerous substances and present antigens to other cells of the immune system. Monocytes are split into two groups: a circulating group and a marginal group (approximately 70% are in the marginal group). Most monocytes leave circulation to travel to tissues and organs. When monocytes leave circulation they transform into macrophages.[12] Monocytes also serve as precursors to dendritic cells.[13]
Macrophages
Macrophages derive from monocytes, granulocyte-monocyte precursors, or from the division of preexisting macrophages. This type of phagocyte does not have granules but contains many lysosomes. Macrophages are found throughout the body in almost all tissues and organs (e.g. microglia cells in the brain and alveolar macrophages in the lungs). A macrophage's location can also determine it size and appearance. Macrophages have many functions: they can phagocytize cell debris and foreign or harmful cells and antibodies (they are frequently seen with cytoplasmic projections that are used for engulfment), they are involved in antigen presentation, and they can even store iron. [23] Macrophages also participate in inflammation through the production of IL-6, TNF-alpha, and IL-1.[24] Macrophages are usually only found in tissue and are rarely seen in circulation. Most macrophages have a lifespan of 3-6 weeks. [23]
Macrophages can be activated so that they can perform functions that cannot be performed by a resting monocyte.[24] Th1 cells are responsible for the activation of macrophages. Th1 cells activate macrophages by signaling with IFN-gamma and displaying the protein CD40 ligand.[25] Other signals include TNF-alpa and lipopolysaccharides from bacteria.[24] The signals then allow the macrophage to effectively kill the microbes that were residing in their phagosomes. Th1 cells can recruit other phagocytes in several ways. They secrete cytokines that act on the bone marrow to stimulate the production of monocytes and neutrophils and they secrete some of the cytokines and chemokines that are responsible for the migration of monocytes and neutrophils out of the blood stream.[25] Th 1 cells come from the differentiation of CD4 T cells once they have responded to antigen in the secondary lymphoid tissues. [24] Macrophages’ NADPH oxidase(an enzyme that plays a role in respiratory bursts)activity increases after activation as well.[26] Activated macrophages also play a more potent role in tumor destruction after activation by producing TNF-alpha, IFN-gamma, nitric oxide, reactive oxygen compounds, cationic proteins, and hydrolytic enzymes.[24]
Neutrophils
Neutrophils participate in phagocytosis of antibody and complement coated antigens. They can also phagocytize damaged cells or cell parts. Neutrophils have a segmented nucleus. This means that they have a nucleus that has several sections; each section is connected by chromatin filaments—neutrophils can have 2-5 segments. Neutrophils do not normally exit the bone marrow until their nucleus has been segmented; but if there is a high need for neutrophils or if there are irregularities in the bone marrow, neutrophil precursors called myelocytes and promyelocytes are released. Neutrophils are also separated between circulating and marginal groups (about 50% of neutrophils are marginated). [27]
Neutrophils can also secrete products that stimulate monocytes and macrophages. Neutrophil secretions increase phagocytosis and the formation of reactive oxygen compounds involved in intracellular killing. [28] Heparin-binding protein and human neutrophil peptides 1-3 have been found to mediate the response to neutrophil secretions. Secretion from the primary granules of neutrophils stimulated the phagocytosis of IgG-coated bacteria. [29]
Dendritic cells

Dendritic cells are specialized antigen-presenting cells that grow long processes or projections called dendrites.[30]. These cells derive from the bone marrow and are present in small quantities in tissues that are in contact with the external environment, mainly the skin (where there is a specialized dendritic cell type called Langerhans cells) and the inner lining of the nose, lungs, stomach and intestines. They can also be found in an immature state in the blood. Once activated, they migrate to the lymphoid tissues where they interact with T cells and B cells to initiate and shape the adaptive immune response.[31] After monocytes have turned into immature dendritic cells, the immature dendritic cells circulate throughout the body. The dendrites help to engulf microbes and other antigen sources in peripheral tissues. [32] [7] Once antigens have been engulfed, they are converted into proteolytic peptides and are attached to MHC class I or II molecules. Following the conversion of antigens into proteolytic peptides, dendritic cells travel to secondary lymphoid organs and mature so that they can present the antigens to T lymphocytes. [7] Dendritic cells when matured can produce other products that stimulate T lymphocytes and help orchestrate the immune response. How effective the immune response controlled be dendritic cells is, depends on their maturity. Maturity can be increased through signals from captured microbes and antigens and other factors in the immune system. Dendritic cells also activate both helper T cells and killer T cells. The activated helper T cells also interact with macrophages and B cells to activate them. In addition, dendritic cells are capable of influencing the type of immune response (whether it be for viruses or something else); when they travel to the lymphoid areas where T cells are held they select the specific T cells for the job. These T cells then differentiate into killer T cells and helper T cells. [32]
Bacterial evasion and resistance
A pathogen is only successful in infecting an organism if it can get past its defenses. Because, of this bacteria have developed many different methods to keep from being engulfed or killed by phagocytes (the first line of defense).[33]
Avoiding contact
There are several ways bacteria avoid contact with phagocytes. First, they may reside in places that phagocytes are not capable of traveling to (such as the urinary bladder and the surface of unbroken skin). Second, bacteria can keep from starting a large inflammatory response as a result of their infection; without an inflammatory response phagocytes cannot respond effectively. Third, bacteria may also keep phagocytes from traveling to the site of infection. Bacteria accomplish this by interfering with chemotaxis. Some strains of Mycobacterium tuberculosis hinder leukocyte chemotaxis; bacteria that fall under the genus Clostridium produce a toxin that inhibits neutrophil migration, as well. Fourth, bacteria avoid contact with phagocytes by tricking the immune system into thinking that the bacteria are “self”. This is demonstrated by Treponema pallidum: It coats its surface with fibronectin.[34]
Avoiding engulfment
Bacteria usually have a component in their cell wall that allows them to resist engulfment by a phagocyte. [33] An example of this is the K5 capsule and the O75 O antigen found on the surface of Escherichia coli used to prevent phagocytosis. [35] Bacteria can also produce a biofilm (a mix of various sugar polymers called exopolysaccharide) on their surface that inhibits phagocytosis. This is seen in Staphylococcus epidermidis: To avoid engulfment, it produces a biofilm composed of poly-N-acetylglucosamine. [36] Some bacteria use a polysaccharide capsule as a shield against phagocytic engulfment. This is done by Streptococcus pneumoniae—there are several types of capsules that are used, all with different levels of protections. [37] Group A streptococci use surface proteins such as M protein and fimbrial proteins to block engulfment. Proteins can also be used to hinder antibody related ingestion. Staphylococcus aureus does this by using Protein A (it attaches ot the Fc receptor to decrease the effectiveness of IgG antibodies).[38]
Survival inside the phagocyte
Bacteria have developed ways to survive inside phagocytes where they are protected from harmful drugs and extracellular bactericidal compounds. However, these bacteria must first get inside the phagocyte and they do this by expressing a protein called invasions (Salmonella and Legionella do this). Legionella pneumophila enters phagocytes by coating its surface with the complement factor C3b. There are many methods of survival. Stopping the fusion of a phagosome and lysosome into a phagolysosome is one method. [33] Legionella pneumophila does this by using a secretion system. These secretions cause the phagosome to fuse with vesicles other than the ones that contain bactericidal compounds. These bacteria also inhibit the trafficking of vesicles and changes the phagosome that they are in. [39] Some bacteria are capable of living inside of the phagolysosome as another means of survival. Staphylococcus aureus does this by producing the enzymes catalase and superoxide dismutase. These enzymes break down bactericidal products (e.g. hydrogen peroxide). [40] Bacteria may also escape from the phagosome before the formation of the phagolysosome as another method of survival. Listeria monocytogenes does this by using a pore forming enzyme called listeriolysin O, and two variants of the bacterial enzyme phospholipase C.[41]
Killing
Bacteria have also developed ways of killing phagocytes.[38] Some of the ways bacteria kill phagocytes before being engulfed include: cytolysins (that form pores in the phagocyte's cell membranes), using streptolysins (this causes a neutrophil’s granules to rupture releasing toxic substances), using leukocidin [42][43] (this also results in the release of the contents in a neutrophil’s granules), and using exotoxins (these toxins can reduce the supply of a phagocyte's ATP, which is needed for phagocytosis). After a bacterium is ingested it may kill the phagocyte by releasing toxins that travel through the phagosome or phagolysosome membrane to target other parts of the cell. [33]
References
- ^ Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G; et al. (2006). "Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion". Immunobiology. 211 (6–8): 487–501. doi:10.1016/j.imbio.2006.06.002. PMID 16920488. Retrieved 2008-11-01.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Langermans, JA (1994-09-14). "Antimicrobial functions of mononuclear phagocytes". Journal of Immunological Methods. 174 (1–2): 185–94. PMID 8083520. Retrieved 2008-11-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i j k Mayer, Gene (2006). "Immunology — Chapter One: Innate (non-specific) Immunity". Microbiology and Immunology On-Line Textbook. USC School of Medicine. Retrieved 2008-11-12.
- ^ "phago-". Dictionary.com. Retrieved 2008-11-12.
- ^ "-cyte". Dictionary.com. Retrieved 2008-11-13.
- ^ Miyasaki, Ken. "Phagocytes-Neutrophils". Retrieved 2008-11-13.
- ^ a b c Guermonprez, P (2002). "Antigen presentation and T cell stimulation by dendritic cells". Annual Review of Immunology. 20: 621–67. PMID 11861614. Retrieved 2008-11-12.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Ilya Mechnikov". The Nobel Foundation. Retrieved 2008-11-28.
- ^ Schmalstieg, FC (2008). "Ilya Ilich Metchnikoff (1845-1915) and Paul Ehrlich (1854-1915): the centennial of the 1908 Nobel Prize in Physiology or Medicine". Journal of medical biography. 16 (2): 96–103. PMID 18463079. Retrieved 2008-11-28.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ May, RC (2001). "Phagocytosis and the actin cytoskeleton". Journal of Cell Science. 114 (6): 119–33. PMID 11228151. Retrieved 2008-11-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Ryter, A (1985). "Relationship between ultrastructure and specific functions of macrophages". Comparative Immunology, Microbiology and Infectious Diseases. 8 (2): 119–33. PMID 3910340. Retrieved 2008-11-13.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b Hess, Charles E. "Monocyte". Retrieved 2008-11-12. Cite error: The named reference "mono" was defined multiple times with different content (see the help page).
- ^ a b Hess, Charles E. "Dendritic Cell". Retrieved 2008-11-12. Cite error: The named reference "dendrite" was defined multiple times with different content (see the help page).
- ^ a b Janeway, Charles A. Induced innate responses to infection. ISBN 978-0-8153-4123-9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zen, K (2005). "Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils". Molecular Biology of the Cell. 16 (6): 2694–703. PMID 15800062. Retrieved 2008-11-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zen, K (2003). "Leukocyte-epithelial interactions". Current Opinion in Cell Biology. 15 (5): 557–64. PMID 14519390. Retrieved 2008-11-12.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Dahlgren, C (1999-12-17). "Respiratory burst in human neutrophils". Journal of Immunological Methods. 232 (1–2): 3–14. PMID 10618505. Retrieved 2008-11-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Shatwell, KP (1996). "NADPH oxidase". The international journal of biochemistry and cell biology. 28 (11): 1191–5. PMID 9022278. Retrieved 2008-12-30.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Klebenoff, SJ (1999). "Myeloperoxidase". Proceedings of the Association of American Physicians. 111 (5): 383–9. PMID 10519157. Retrieved 2008-12-30.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Masek, Katherine S. (2007). Eurekah Bioscience Collection: Macrophage Effector Functions. Landes Bioscience.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Antigen Presenting Cells (APC)". Dalhousie University. Retrieved 2008-11-13.
- ^ Janeway, Charles A. (2007). Immunobiology: Antigen Presentation to T Lymphocytes. Garland Science. ISBN 978-0-8153-4123-9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Hess, Charles. "Histiocyte". University of Virginia Health System. Retrieved 2008-11-14.
- ^ a b c d e Bowers, William (2006). "Immunology -Chapter Thirteen: Immunoregulation". Microbiology and Immunology On-Line Textbook. USC School of Medicine. Retrieved 2008-11-14. Cite error: The named reference "USCmac" was defined multiple times with different content (see the help page).
- ^ a b Alberts, Bruce (2002). Molecular Biology of the Cell; Fourth Edition. New York and London: Garland Science. ISBN 0-8153-3218-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ryter, A (1985). "Relationship between ultrastructure and specific functions of macrophages". Comparative Immunology, Microbiology and Infectious Diseases. 8 (2): 119–33. PMID 3910340. Retrieved 2008-11-14.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Hess, Charles. "Segmented Neutrophil". University of Virginia Health System. Retrieved 2008-11-14.
- ^ Soehnlein, O (2008). "Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages". Clinical and Experimental Immunology. 151 (1): 139–45. PMID 17991288. Retrieved 2008-11-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Soehnlein, O (2008). "Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages". The Journal of Clinical Investigation. 118 (10): 3491–502. PMID 18787642. Retrieved 2008-11-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Steinman RM, Cohn ZA (1973). "Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution". J. Exp. Med. 137 (5): 1142–62. doi:10.1084/jem.137.5.1142. PMID 4573839.
- ^ Sallusto F, Lanzavecchia A (2002). "The instructive role of dendritic cells on T-cell responses". Arthritis Res. 4 Suppl 3: S127–32. PMID 12110131.
- ^ a b Steinman, Ralph. "Dendritic Cells". The Rockefeller University. Retrieved 2008-11-16.
- ^ a b c d Todar, Kenneth. "MECHANISMS OF BACTERIAL PATHOGENICITY: Bacterial Defense Against Phagocytes". 2008. Retrieved 2008-12-10. Cite error: The named reference "chicken" was defined multiple times with different content (see the help page).
- ^ Celli J, Finlay BB (2002). "Bacterial avoidance of phagocytosis". Trends Microbiol. 10 (5): 232–7. PMID 11973157. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Burns, SM (1999). "Loss of resistance to ingestion and phagocytic killing by O(-) and K(-) mutants of a uropathogenic Escherichia coli O75:K5 strain". Infection and immunity. 67 (8): 3757–62. PMID 10417134. Retrieved 2008-12-10.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vuong, C (2004-12-24). "A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence". The journal of biological chemistry. 279 (52): 54881–6. PMID 15501828. Retrieved 2008-12-10.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Melin, M (2008-12-1). "Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B". Infection and immunity. PMID 19047408. Retrieved 2008-12-10.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Foster TJ (2005). "Immune evasion by staphylococci". Nat. Rev. Microbiol. 3 (12): 948–58. doi:10.1038/nrmicro1289. PMID 16322743. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help) Cite error: The named reference "pmid16322743" was defined multiple times with different content (see the help page). - ^ Masek, Katherine S. (2007). Eurekah Bioscience Collection: Evasion of Phagosome Lysosome Fusion and Establishment of a Replicative Organelle by the Intracellular Pathogen Legionella pneumophila. Landes Bioscience.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Das, D (2008-12-1). "Intracellular survival of Staphylococcus aureus: correlating production of catalase and superoxide dismutase with levels of inflammatory cytokines". Infection and immunity. 57 (7): 340–9. PMID 18607538. Retrieved 2008-12-10.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hara H, Kawamura I, Nomura T, Tominaga T, Tsuchiya K, Mitsuyama M (2007). "Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement". Infect. Immun. 75 (8): 3791–801. doi:10.1128/IAI.01779-06. PMC 1951982. PMID 17517863. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Datta V, Myskowski SM, Kwinn LA, Chiem DN, Varki N, Kansal RG, Kotb M, Nizet V (2005). "Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection". Mol. Microbiol. 56 (3): 681–95. doi:10.1111/j.1365-2958.2005.04583.x. PMID 15819624. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Iwatsuki K, Yamasaki O, Morizane S, Oono T (2006). "Staphylococcal cutaneous infections: invasion, evasion and aggression". J. Dermatol. Sci. 42 (3): 203–14. doi:10.1016/j.jdermsci.2006.03.011. PMID 16679003. Retrieved 2008-12-12.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- Phagocytes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
