Ultraviolet: Difference between revisions
mNo edit summary |
|||
| Line 1: | Line 1: | ||
{{redirect|UV|other uses of UV|UV (disambiguation)}} |
|||
[[fa:فرابنفش]] |
|||
[[ |
{{dablink|For the movie, see [[Ultraviolet (film)]].}} |
||
[[gl:Ultravioleta]]Wikipedia is sustained by people like you. Please donate today.Skin |
|||
From Wikipedia, the free encyclopedia |
|||
Jump to: navigation, search |
|||
For other uses, see Skin (disambiguation). |
|||
This article needs additional citations for verification. |
|||
Please help improve this article by adding reliable references. Unsourced material may be challenged and removed. (January 2008) |
|||
It has been suggested that Skin type be merged into this article or section. (Discuss) |
|||
Skin layers: epidermis, dermis, and subcutis, showing a hair follicle, sweat gland & sebaceous gland. |
|||
Skin pores with benign lentigo on a shaved female human leg.The skin is the outer covering of the body. In humans, it is the largest organ of the integumentary system made up of multiple layers of epithelial tissues, and guards the underlying muscles, bones, ligaments and internal organs.[1] Skin of a different nature exists in amphibians, reptiles, birds.[2] Human skin is not unlike that of most other mammals except that it is not protected by a pelt and appears hairless though in fact nearly all human skin is covered with hair follicles. The adjective cutaneous literally means "of the skin" (from Latin cutis, skin). |
|||
{{pp-move-indef}} |
|||
Because it interfaces with the environment, skin plays a key role in protecting (the body) against pathogens[3] and excessive water loss.[4] Its other functions are insulation, temperature regulation, sensation, synthesis of vitamin D, and the protection of vitamin B folates. Severely damaged skin will try to heal by forming scar tissue. This is often discolored and depigmented. |
|||
[[Image:Blue sun.jpg|thumb|right|[[False-color]] image of the Sun's [[corona]] as seen in deep ultraviolet by the [[Extreme ultraviolet Imaging Telescope]]]] |
|||
In humans, skin pigmentation varies among populations, and skin type can range from dry to oily. Such skin variety provides a rich and diverse habit for bacteria which number roughly a 1000 species from 19 phyla.[5][6] |
|||
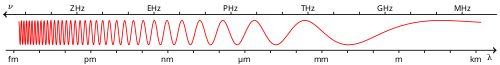
'''Ultraviolet''' ('''UV''') light is [[electromagnetic radiation]] with a [[wavelength]] shorter than that of [[visible light]], but longer than [[x-ray]]s, in the range 10 [[nanometer|nm]] to 400 nm, and energies from 3 [[Electron volt|eV]] to 124 eV. It is so named because the spectrum consists of electromagnetic waves with frequencies higher than those that [[human]]s identify as the color [[Violet (color)|violet]]. |
|||
UV light is found in [[sunlight]] and is emitted by [[Electric arc discharge|electric arcs]] and specialized lights such as [[black light]]s. As an [[ionizing radiation]] it can cause chemical reactions, and causes many substances to glow or [[Fluorescence|fluoresce]]. Most people are aware of the effects of UV through the painful condition of [[sunburn]], but the UV spectrum has many other effects, both beneficial and damaging, on human health. |
|||
Contents [hide] |
|||
1 Skin components |
|||
2 Functions |
|||
3 Hygiene and skin care |
|||
4 Aging |
|||
5 Disease |
|||
6 Variability in skin tone |
|||
6.1 Skin types |
|||
7 Skin flora |
|||
8 Animal skin products |
|||
9 Skin layers |
|||
9.1 Epidermis |
|||
9.1.1 Components |
|||
9.1.2 Layers |
|||
9.1.3 Sublayers |
|||
9.2 Dermis |
|||
9.2.1 Papillary region |
|||
9.2.2 Reticular region |
|||
9.3 Hypodermis |
|||
10 See also |
|||
11 References |
|||
== Discovery == |
|||
The discovery of UV radiation was intimately associated with the observation that [[Silver halide|silver salts]] darken when exposed to sunlight. In 1801 the German physicist [[Johann Wilhelm Ritter]] made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum were especially effective at darkening [[silver chloride]]-soaked paper. He called them "de-oxidizing rays" to emphasize their [[Reactivity (chemistry)|chemical reactivity]] and to distinguish them from "heat rays" at the other end of the visible spectrum. The simpler term "chemical rays" was adopted shortly thereafter, and it remained popular throughout the 19th century. The terms chemical and heat rays were eventually dropped in favor of ultraviolet and [[infrared]] [[radiation]], respectively.<ref name="hockberger"> |
|||
{{Citation |
|||
| last = Hockberger |
|||
| first = P. E. |
|||
| title = A history of ultraviolet photobiology for humans, animals and microorganisms |
|||
| journal = Photochem. Photobiol. |
|||
| volume = 76 |
|||
| issue = |
|||
| pages = 561–579 |
|||
| year = 2002 |
|||
| url = http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve%5C&db=pubmed%5C&dopt=Abstract%5C&list_uids=12511035 |
|||
| doi =10.1562/0031-8655(2002)076<0561:AHOUPF>2.0.CO;2 |
|||
}} </ref> |
|||
The discovery of the ultraviolet radiation below 200 nm, named vacuum ultraviolet because it is strongly absorbed by air, was made in 1893 by the German physicist [[Victor Schumann]].<ref name="Lyman"> The ozone layer protects humans from this. |
|||
[edit] Skin components |
|||
{{Citation |
|||
See also: Skin layers |
|||
| last = Lyman |
|||
Skin has pigmentation, or melanin, provided by melanocytes, which absorb some of the potentially dangerous ultraviolet radiation (UV) in sunlight. It also contains DNA-repair enzymes that help reverse UV damage, and people who lack the genes for these enzymes suffer high rates of skin cancer. One form predominantly produced by UV light, malignant melanoma, is particularly invasive, causing it to spread quickly, and can often be deadly. Human skin pigmentation varies among populations in a striking manner. This has led to the classification of people(s) on the basis of skin color.[7] |
|||
| first = T. |
|||
| title = Victor Schumann |
|||
| journal = Astrophysical Journal |
|||
| volume = 38 |
|||
| issue = |
|||
| pages = 1–4 |
|||
| year = 1914 |
|||
| url = http://articles.adsabs.harvard.edu/full/1914ApJ....39....1L |
|||
| doi =10.1086/142050 |
|||
}} </ref> |
|||
== Origin of term == |
|||
Mammalian skin often contains hairs, which in sufficient density is called fur. The hair mainly serves to augment the insulation the skin provides, but can also serve as a secondary sexual characteristic or as camouflage. On some animals, the skin is very hard and thick, and can be processed to create leather. Reptiles and fish have hard protective scales on their skin for protection, and birds have hard feathers, all made of tough β-keratins. Amphibian skin is not a strong barrier to passage of chemicals and is often subject to osmosis. A frog sitting in an anesthetic solution could quickly go to sleep. |
|||
The name means "beyond violet" (from [[Latin]] ''ultra'', "beyond"), [[violet (colour)|violet]] being the [[color]] of the shortest wavelengths of visible light. UV light has a shorter wavelength than that of violet light. |
|||
== Subtypes == |
|||
The skin is the largest organ in the human body. For the average adult human, the skin has a surface area of between 1.5-2.0 square meters (16.1-21.5 sq ft.), most of it is between 2-3 mm (0.10 inch) thick. The average square inch (6.5 cm²) of skin holds 650 sweat glands, 20 blood vessels, 60,000 melanocytes, and more than a thousand nerve endings. |
|||
The electromagnetic spectrum of ultraviolet light can be subdivided in a number of ways. The draft ISO standard on determining solar irradiances (ISO-DIS-21348)<ref>{{cite web | title = ISO 21348 Process for Determining Solar Irradiances | url = http://www.spacewx.com/ISO_solar_standard.html}}</ref> describes the following ranges: |
|||
{|class="wikitable" border="1" |
|||
|- |
|||
!Name |
|||
!Abbreviation |
|||
![[Wavelength]] range in [[nanometers]] |
|||
!Energy per photon |
|||
|- |
|||
|Ultraviolet A, long wave, or [[black light]] |
|||
|UVA |
|||
|400 nm–320 nm |
|||
|3.10–3.94 eV |
|||
|- |
|||
|'''Near''' |
|||
|NUV |
|||
|400 nm–300 nm |
|||
|3.10–4.13 eV |
|||
|- |
|||
|Ultraviolet B or medium wave |
|||
|UVB |
|||
|320 nm–280 nm |
|||
|3.94–4.43 eV |
|||
|- |
|||
|'''Middle''' |
|||
|MUV |
|||
|300 nm–200 nm |
|||
|4.13–6.20 eV |
|||
|- |
|||
|Ultraviolet C, short wave, or [[Ultraviolet germicidal irradiation|germicidal]] |
|||
|UVC |
|||
|280 nm–100 nm |
|||
|4.43–12.4 eV |
|||
|- |
|||
|'''Far''' |
|||
|FUV |
|||
|200 nm–122 nm |
|||
|6.20–10.2 eV |
|||
|- |
|||
|'''Vacuum''' |
|||
|VUV |
|||
|200 nm–10 nm |
|||
|6.20–124 eV |
|||
|- |
|||
|'''Extreme''' |
|||
|[[EUV]] |
|||
|121 nm–10 nm |
|||
|10.2–124 eV |
|||
|} |
|||
In [[photolithography]] and [[laser]] technology, the term deep ultraviolet or DUV refers to wavelengths below 300 nm. "Vacuum UV" is so named because it is absorbed strongly by [[air]] and is therefore used in a vacuum. In the long-wave limit of this region, roughly 150–200 nm, the principal absorber is the [[oxygen]] in air. Work in this region can be performed in an oxygen free atmosphere, pure nitrogen being commonly used, which avoids the need for a vacuum chamber. |
|||
[edit] Functions |
|||
Skin performs the following functions: |
|||
See [[1 E-7 m]] for a list of objects of comparable sizes. |
|||
Protection: an anatomical barrier from pathogens and damage between the internal and external environment in bodily defense; Langerhans cells in the skin are part of the adaptive immune system.[4][3] |
|||
Sensation: contains a variety of nerve endings that react to heat and cold, touch, pressure, vibration, and tissue injury; see somatosensory system and haptics. |
|||
Heat regulation: the skin contains a blood supply far greater than its requirements which allows precise control of energy loss by radiation, convection and conduction. Dilated blood vessels increase perfusion and heat loss while constricted vessels greatly reduce cutaneous blood flow and conserve heat. Erector pili muscles are significant in animals. |
|||
Control of evaporation: the skin provides a relatively dry and semi-impermeable barrier to fluid loss.[4] Loss of this function contributes to the massive fluid loss in burns. |
|||
Aesthetics and communication: others see our skin and can assess our mood, physical state and attractiveness. |
|||
Storage and synthesis: acts as a storage center for lipids and water, as well as a means of synthesis of vitamin D by action of UV on certain parts of the skin. |
|||
Excretion: sweat contains urea, however its concentration is 1/130th that of urine, hence excretion by sweating is at most a secondary function to temperature regulation. |
|||
Absorption: Oxygen, nitrogen and carbon dioxide can diffuse into the epidermis in small amounts, some animals using their skin for their sole respiration organ. In addition, medicine can be administered through the skin, by ointments or by means of adhesive patch, such as the nicotine patch or iontophoresis. The skin is an important site of transport in many other organisms. |
|||
Water resistance: The skin acts as a water resistant barrier so essential nutrients aren't washed out of the body. |
|||
== Black light == |
|||
[edit] Hygiene and skin care |
|||
{{main|Black light}} |
|||
See also: Exfoliation (cosmetology) |
|||
A black light, or [[Wood's light]], is a lamp that emits long wave UV radiation and very little visible light. Commonly these are referred to as simply a "UV light". Fluorescent black lights are typically made in the same fashion as normal fluorescent lights except that only one phosphor is used and the normally clear glass envelope of the bulb may be replaced by a deep-bluish-purple glass called [[Wood's glass]], a nickel-oxide–doped glass, which blocks almost all visible light above 400 nanometers. The color of such lamps is often referred to in the trade as "blacklight blue" or "BLB." This is to distinguish these lamps from "bug zapper" blacklight ("BL") lamps that don't have the blue Wood's glass. The phosphor typically used for a near 368 to 371 nanometer emission peak is either europium-doped strontium fluoroborate (SrB4O7F:Eu2+) or europium-doped strontium borate (SrB4O7:Eu2+) while the phosphor used to produce a peak around 350 to 353 nanometers is lead-doped barium silicate (BaSi2O5:Pb+). "Blacklight Blue" lamps peak at 365 nm. |
|||
The skin supports its own ecosystems of microorganisms, including yeasts and bacteria, which cannot be removed by any amount of cleaning. Estimates place the number of individual bacteria on the surface of one square inch (6.5 square cm) of human skin at 50 million though this figure varies greatly over the average 20 square feet (1.9 m2) of human skin. Oily surfaces, such as the face, may contain over 500 million bacteria per square inch (6.5 cm²). Despite these vast quantities, all of the bacteria found on the skin's surface would fit into a volume the size of a pea.[8] In general, the microorganisms keep one another in check and are part of a healthy skin. When the balance is disturbed, there may be an overgrowth and infection, such as when antibiotics kill microbes, resulting in an overgrowth of yeast. The skin is continuous with the inner epithelial lining of the body at the orifices, each of which supports its own complement of microbes. |
|||
While "black lights" do produce light in the UV range, their spectrum is confined to the longwave UVA region. Unlike UVB and UVC, which are responsible for the direct DNA damage that leads to skin cancer, black light is limited to lower energy, longer waves and does not cause sunburn. However, UVA is capable of causing damage to collagen fibers and destroying vitamin A in skin. |
|||
Proper skin hygiene is important because unclean skin favors the development of pathogenic organisms. The dead cells that continually slough off the epidermis mix with the secretions of the sweat and sebaceous glands and the dust found on the skin form a filthy layer on its surface. If not washed away, the slurry of sweat and sebaceous secretions mixed with dirt and dead skin is decomposed by bacterial flora, producing a foul smell. Functions of the skin are disturbed when it is excessively dirty; it becomes more easily damaged, the release of antibacterial compounds decreases, and dirty skin is more prone to develop infections. |
|||
A black light may also be formed by simply using Wood's glass instead of clear glass as the envelope for a common incandescent bulb. This was the method used to create the very first black light sources. Though it remains a cheaper alternative to the fluorescent method, it is exceptionally inefficient at producing UV light (less than 0.1% of the input power) owing to the [[black body]] nature of the incandescent light source. Incandescent UV bulbs, due to their inefficiency, may also become dangerously hot during use. More rarely still, high power (hundreds of watts) mercury vapor black lights can be found which use a UV emitting phosphor and an envelope of Wood's glass. These lamps are used mainly for theatrical and concert displays and also become very hot during normal use. |
|||
Cosmetics should be used carefully on the skin because these may cause allergic reactions. Each season requires suitable clothing in order to facilitate the evaporation of the sweat. Sunlight, water and air play an important role in keeping the skin healthy. |
|||
Some UV fluorescent bulbs specifically designed to attract insects for use in bug zappers use the same near-UV emitting phosphor as normal blacklights, but use plain glass instead of the more expensive Wood's glass. Plain glass blocks less of the visible mercury emission spectrum, making them appear light blue to the naked eye. These lamps are referred to as "blacklight" or "BL" in most lighting catalogs. |
|||
Oily skin is caused by over-active sebaceous glands, that produce a substance called sebum, a naturally healthy skin lubricant.[1] When the skin produces excessive sebum, it becomes heavy and thick in texture. Oily skin is typified by shininess, blemishes and pimples.[1] The oily-skin type is not necessarily bad, since such skin is less prone to wrinkling, or other signs of aging,[1] because the oil helps to keep needed moisture locked into the epidermis (outermost layer of skin). |
|||
Ultraviolet light can also be generated by some [[Light-emitting diode#Ultraviolet and blue LEDs|light-emitting diodes]]. |
|||
The negative aspect of the oily-skin type is that oily complexions are especially susceptible to clogged pores, blackheads, and buildup of dead skin cells on the surface of the skin.[1] Oily skin can be sallow and rough in texture and tends to have large, clearly visible pores everywhere, except around the eyes and neck.[1] |
|||
== Natural sources of UV == |
|||
The goal of treating oily skin is to remove excess surface sebum without complete removal of skin lipids.[1] Severe degreasing treatment can foster an actual worsening of sebum secretion, which defeats the aim of the cleansing.[1] A method of cleansing oily skin is to cleanse with a natural face cleanser formulated especially for oily skin. The cleansers pH should be 4.5 - 5.5, since the skin's pH value is approximately 5.4. Gel cleansers work best on oily skin.[1] (see: surfactant) Oily skin products should contain very little natural oils. They should not contain waxes or other synthetic lipid agents that could aggravate the oily condition of the skin. A toning lotion should also be natural and have a pH of 4.5-5.5 and formulated especially to help balance and hydrate oily skin. Some cleansing products have lower concentrations of hydroxy acids, which remove dead cells from the upper levels of the stratum corneum.[1] Those products should be used on a regular basis to work adequately.[1] A light moisturizer may be included in a hydoxy acid product to counteract any drying effects of the cleanser.[1] People with oily skin should use a moisturizer with humectants and a clay masques containing bentonite clay twice a week. |
|||
The [[Sun]] emits ultraviolet radiation in the UVA, UVB, and UVC bands, but because of absorption in the [[Earth's atmosphere|atmosphere's]] [[ozone layer]], 98.7% of the ultraviolet radiation that reaches the Earth's surface is UVA. (Some of the UVB and UVC radiation is responsible for the generation of the ozone layer.) |
|||
Ordinary glass is partially [[transparent]] to UVA but is [[opaque]] to shorter wavelengths while [[fused quartz|Silica or quartz glass]], depending on quality, can be transparent even to vacuum UV wavelengths. Ordinary window glass passes about 90% of the light above 350 nm, but blocks over 90% of the light below 300 nm.<ref>{{cite web | title = Soda Lime Glass Transmission Curve | url = http://www.sinclairmfg.com/datasheets/sodalimecurve.htm}}</ref><ref>{{cite web | title = B270-Superwite Glass Transmission Curve | url = http://www.pgo-online.com/intl/katalog/curves/B270_kurve.html}}</ref><ref>{{cite web | title = Selected Float Glass Transmission Curve | url = http://www.pgo-online.com/intl/katalog/curves/whitefl_kurve.html}}</ref> |
|||
The onset of vacuum UV, 200 nm, is defined by the fact that ordinary air is opaque at shorter wavelengths. This opacity is due to the strong absorption of light |
|||
[edit] Aging |
|||
of these wavelengths by oxygen in the air. Pure nitrogen (less than about 10 ppm oxygen) is transparent to wavelengths in the range of about 150–200 nm. This has wide practical significance now that semiconductor manufacturing processes are using wavelengths shorter than 200 nm. By working in oxygen-free gas, the equipment does not have to be built to withstand the pressure differences required to work in a vacuum. Some other scientific instruments, such as [[circular dichroism]] spectrometers, are also commonly nitrogen purged and operate in this spectral region. |
|||
For more details on this topic, see senescence. |
|||
A typical rash |
|||
Skin infected with ScabiesAs skin ages, it becomes thinner and more easily damaged. Intensifying this effect is the decreasing ability of skin to heal itself as a person ages. |
|||
Extreme UV is characterized by a transition in the physics of interaction with matter: wavelengths longer than about 30 nm interact mainly with the chemical [[valence electrons]] of matter, while wavelengths shorter than that interact mainly with inner shell electrons and nuclei. The long end of the EUV/XUV spectrum is set by a prominent [[helium|He<sup>+</sup>]] [[spectral line]] at 30.4 nm. XUV is strongly absorbed by most known materials, but it is possible to synthesize [[multilayer optics]] that reflect up to about 50% of XUV radiation at [[normal incidence]]. This technology has been used to make telescopes for [[Sun|solar imaging]]; it was pioneered by the [[NIXT]] and [[MSSTA]] sounding rockets in the 1990s; (current examples are [[Solar and Heliospheric Observatory|SOHO]]/EIT and [[TRACE]]) and for [[nanolithography]] (printing of traces and devices on [[microchips]]). |
|||
Skin aging is caused by the fall in elasticity. Aging skin also receives less blood flow and lower gland activity. |
|||
== Human health-related effects of UV radiation == |
|||
Cortisol causes degradation of skin collagen[9], accelerating skin aging.[10] |
|||
=== Beneficial effects === |
|||
==== Vitamin D ==== |
|||
The Earth's atmosphere blocks UV radiation from penetrating through the atmosphere by 98.7%. A positive effect of UVB exposure is that it induces the production of [[vitamin D]] in the skin. It has been estimated that tens of thousands of premature deaths occur in the United States annually from a range of cancers due to vitamin D deficiency.<ref>{{cite paper | title = An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation | author = Grant, W. B. | publisher = Cancer Volume 94, Issue 6, pp. 1867-1875 |date=2002 | url = http://www3.interscience.wiley.com/cgi-bin/abstract/91016211/ABSTRACT}}</ref> Another effect of vitamin D deficiency is poor absorption of [[Calcium metabolism|calcium]] which can lead to bone diseases. |
|||
Some studies show most people get adequate Vitamin D through food and incidental exposure.<ref name="NPR" /> |
|||
Many countries have [[Food fortification|fortified]] certain foods with Vitamin D to prevent deficiency. Eating fortified foods or taking a [[dietary supplement]] pill is usually preferred to UVB exposure, due to the increased risk of skin cancer from UV radiation.<ref name="NPR">[http://www.npr.org/templates/story/story.php?storyId=4717388 The Science of Sun Protection], Talk of the Nation Science Friday, 24 June 2005. Vitamin D pills recommended over sun exposure, but most people in Australia and Canada get enough Vitamin D by incidental exposure, studies show.</ref> |
|||
[edit] Disease |
|||
For more details on this topic, see skin disease. |
|||
Dermatology is the branch of medicine that deals with conditions of the skin.[11] |
|||
==== Aesthetics ==== |
|||
Too little UVB radiation leads to a lack of Vitamin D. Too much UVB radiation leads to [[direct DNA damage]]s and sunburn. An appropriate amount of UVB (which varies according to [[human skin color|skin color]]) leads to a limited amount of direct DNA damage. This is recognized and repaired by the body. Then the melanin production is increased which leads to a long lasting tan. This tan occurs with a 2 day lag phase after irradiation, but it is much less harmful and long lasting than the one obtained from UVA. However some tanning lotions and sprays available in the market don't require UV exposure. |
|||
==== Medical applications ==== |
|||
[edit] Variability in skin tone |
|||
Ultraviolet radiation has other medical applications, in the treatment of skin conditions such as [[psoriasis]] and [[vitiligo]]. UVA radiation can be used in conjunction with psoralens ([[PUVA]] treatment). UVB radiation is ''rarely'' used in conjunction with [[psoralens]]. In cases of psoriasis and vitiligo, UV light with wavelength of 311 nm is most effective.{{Fact|date=April 2007}} |
|||
Individuals with ancestors from different parts of the world can have highly visible differences in skin pigmentation. Individuals with sub-Saharan African ancestry (black people) tend towards darker skin, while those of Northern European descent (white people) have paler skin. Between these extremes are individuals of Asian, South-East Asian, Native American, Middle Eastern, Polynesian and Melanesian descent. |
|||
=== Harmful effects === |
|||
The skin of black people has more variation in color from one part of the body to another than does the skin of other racial groups, particularly the palms of the hands and soles of the feet. Part of this is the result of the variations in the thickness of the skin or different parts of the body. The thicker the skin, the more layers of cell with melanin in them, and the darker the color.[12] In addition, these parts of the body do not have melanin-producing cells. |
|||
An overexposure to UVB radiation can cause [[sunburn]] and some forms of skin cancer. In humans, prolonged exposure to solar UV radiation may result in acute and chronic [[health effect]]s on the [[skin]], eye, and [[immune system]].<ref>{{cite web | title = Health effects of UV radiation | url = http://www.who.int/uv/health/en/}}</ref> However the most deadly form - malignant melanoma - is mostly caused by the [[indirect DNA damage]] (free radicals and oxidative stress). This can be seen from the absence of a UV-signature mutation in 92% of all melanoma.<ref name=Davies> {{cite journal |author=Davies H.; Bignell G. R.; Cox C.; |year= 2002 |month=6 |title= Mutations of the BRAF gene in human cancer |journal= Nature |volume= 417 |issue= |pages=949–954 |id= |url= http://www.nature.com/nature/journal/v417/n6892/full/nature00766.html | doi = 10.1038/nature00766}}</ref> |
|||
UVC rays are the highest energy, most dangerous type of ultraviolet light. Little attention has been given to UVC rays in the past since they are filtered out by the atmosphere. However, their use in equipment such as pond [[Sterilization (microbiology)|sterilization]] units may pose an exposure risk, if the lamp is switched on outside of its enclosed pond sterilization unit. |
|||
Darker skin hinders UVA rays from penetrating. Because UVA degrades folate (a B vitamin) and is required for vitamin D synthesis, people with darker skin tones are more susceptible to deficiencies of these vitamins. |
|||
[[Image:DNA UV mutation.gif|thumb|right|Ultraviolet photons harm the [[DNA]] molecules of living organisms in different ways. In one common damage event, adjacent [[Thymine]] bases bond with each other, instead of across the "ladder". This makes a bulge, and the distorted DNA molecule does not function properly.]] |
|||
==== Skin ==== |
|||
{{cquote2 | Ultraviolet (UV) irradiation present in sunlight is an environmental human [[carcinogen]]. The toxic effects of UV from natural sunlight and therapeutic artificial lamps are a major concern for human health. The major acute effects of UV irradiation on normal human skin comprise sunburn inflammation [[erythema]], [[sun tanning|tanning]], and local or systemic [[immunosuppression]]. | Matsumura and Ananthaswamy | (2004)<ref> |
|||
Skin can be classified based on its reaction to ultraviolet radiation:[13] |
|||
{{citation |
|||
| title = Toxic effects of ultraviolet radiation on the skin |
|||
| last = Matsumu |
|||
| first = Y. |
|||
| last2 = Ananthaswamy |
|||
| first2 = H. N. |
|||
| year = 2004 |
|||
| journal = Toxicology and Applied Pharmacology |
|||
| volume = 195 |
|||
| issue = 3 |
|||
| pages = 298–308 |
|||
| url = http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15020192 |
|||
| doi = 10.1016/j.taap.2003.08.019 |
|||
}}</ref> |
|||
}} |
|||
UVA, UVB and UVC can all damage [[collagen]] fibers and thereby accelerate aging of the skin. Both UVA and UVB destroy vitamin A in skin which may cause further damage.<ref> |
|||
{{citation |
|||
|last = Torma |
|||
|first = H |
|||
|last2 = Berne |
|||
|first2 = B |
|||
|last3 = Vahlquist |
|||
|first3 = A |
|||
|journal = Acta Derm. Venereol. |
|||
|date=1988 |
|||
|volume = 68 |
|||
|issue = 4 |
|||
|pages = 291--299 |
|||
|title = UV irradiation and topical vitamin A modulate retinol esterification in hairless mouse epidermis |
|||
|url = http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=2459873&dopt=AbstractPlus}} |
|||
</ref> |
|||
In the past, UVA was considered less harmful, but today it is known that it can contribute to skin cancer via indirect DNA damage (free radicals and reactive oxygen species). It penetrates deeply but it does not cause [[sunburn]]. |
|||
UVA does not damage DNA directly like UVB and UVC, but it can generate highly reactive chemical intermediates, such as hydroxyl and oxygen radicals, which in turn can damage DNA. Because it does not cause reddening of the skin (erythema) it cannot be measured in [[Sunscreen#Sun protection factor|SPF]] testing. There is no good clinical measurement for blockage of UVA radiation, but it is important that [[sunscreen]] block both UVA and UVB. Some scientists blame the absence of UVA filters in [[sunscreen]]s for the higher melanoma-risk that was found for sunscreen users.<ref name=Autier> {{cite journal |author=Autier P; Dore J F; Schifflers E; et al. |title=Melanoma and use of sunscreens: An EORTC case control study in Germany, Belgium and France |url= |journal=Int. J. Cancer |volume=61 |issue= |pages=749–755 |year=1995| doi = 10.1002/ijc.2910610602}}</ref> |
|||
[[Image:Erythemal action spectrum.svg|thumb|right|The reddening of the skin due to the action of sunlight depends both on the amount of sunlight and on the sensitivity of the skin ("erythemal action spectrum") over the UV spectrum.]] |
|||
Type Definition Description |
|||
I Always burns but never tans Pale, Fair, Freckles |
|||
II Usually burns, sometimes tans Fair |
|||
III May burn, usually tans Light Brown |
|||
IV Rarely burns, always tans Olive brown |
|||
V Moderate constitutional pigmentation Brown |
|||
VI Marked constitutional pigmentation Black |
|||
UVB light can cause direct DNA damage. The radiation [[excited state|excites]] DNA molecules in skin cells, causing aberrant [[covalent bond]]s to form between adjacent [[cytosine]] bases, producing a [[dimer]]. When DNA polymerase comes along to replicate this strand of DNA, it reads the dimer as "AA" and not the original "CC". This causes the DNA replication mechanism to add a "TT" on the growing strand. This is a [[mutation]], which can result in [[cancer]]ous growths and is known as a "classical C-T mutation". The mutations that are caused by the direct DNA damage carry a UV signature mutation that is commonly seen in skin [[cancer]]s. The [[mutagen]]icity of UV radiation can be easily observed in [[bacterium|bacteria]] cultures. This cancer connection is one reason for concern about [[ozone depletion]] and the ozone hole. UVB causes some damage to collagen but at a very much slower rate than UVA. |
|||
As a defense against UV radiation, the amount of the brown pigment [[melanin]] in the skin increases when exposed to moderate (depending on [[human skin color|skin type]]) levels of radiation; this is commonly known as a [[sun tan]]. The purpose of melanin is to absorb UV radiation and dissipate the energy as harmless heat, blocking the UV from damaging skin tissue. UVA gives a quick tan that lasts for days by oxidizing melanin that was already present and triggers the release of the [[melanin]] from melanocytes. UVB yields a tan that takes roughly 2 days to develop because it stimulates the body to produce more melanin. The photochemical properties of melanin make it an excellent [[photoprotection|photoprotectant]]. However, sunscreen chemicals can not dissipate the energy of the excited state as efficiently as melanin and therefore the penetration of sunscreen ingredients into the lower layers of the skin is increasing the amount of [[free radical]]s and reactive oxygen species ([[ROS]]).<ref name="Hanson">{{cite journal |author=Hanson Kerry M.; Gratton Enrico; Bardeen Christopher J. |title=Sunscreen enhancement of UV-induced reactive oxygen species in the skin |doi=10.1016/j.freeradbiomed.2006.06.011| journal=Free Radical Biology and Medicine |volume=41 |issue=8 |pages=1205–1212 |year=2006 }}</ref> |
|||
[edit] Skin flora |
|||
Main article: Skin flora |
|||
The human skin is a rich environment for microbes.[5][6] Around 1000 species of bacteria from 19 bacterial phyla have been found. Most come from only four phyla: Actinobacteria (51.8%), Firmicutes (24.4%), Proteobacteria (16.5%), and Bacteroidetes (6.3%). Propionibacteria and Staphylococci species were them main species in sebaceous areas. There are three main ecological areas: moist, dry and sebaceous. In moist places on the body Corynebacteria together with Staphylococci dominate. In dry areas, there is a mixture of species but dominated by b-Proteobacteria and Flavobacteriales. Ecologically, sebaceous areas had greater species richness than moist and dry one. The areas with least similarity between people in species were the spaces between fingers, the spaces between toes, axillae, and umbilical cord stump. Most similarly were beside the nostril, nares (inside the nostril), and on the back. |
|||
[[Sunscreen]] prevents the direct DNA damage which causes sunburn. Most of these products contain an [[sunscreen#sun protection factor|SPF rating]] to show how well they block UVB rays. The SPF rating, however, offers no data about UVA protection. In the US, the [[Food and Drug Administration (United States)|Food and Drug Administration]] is considering adding a star rating system to show UVA protection. A similar system is already used in some European countries.{{Fact|date=April 2009}} |
|||
Reflecting upon the diversity of the human skin researchers on the human skin microbiome have observed: "hairy, moist underarms lie a short distance from smooth dry forearms, but these two niches are likely as ecologically dissimilar as rainforests are to deserts."[5] |
|||
Some sunscreen lotions now include compounds such as [[titanium dioxide]] which helps protect against UVA rays. Other UVA blocking compounds found in sunscreen include [[zinc oxide]] and [[avobenzone]]. [[Cantaloupe]] extract, rich in the compound [[superoxide dismutase]] (SOD), can be bound with [[gliadin]] to form [[glisodin]], an orally-effective protectant against UVB radiation. There are also naturally occurring compounds found in rainforest plants that have been known to protect the skin from UV radiation damage, such as the fern ''[[Phlebodium aureum]]''. |
|||
The NIH has been launched the Human Microbiome Project to characterize the human microbiota which includes that on the skin and the role of this microbiome in health and disease.[14] |
|||
===== Sunscreen safety debate ===== |
|||
{{main|Sunscreen controversy}} |
|||
Medical organizations recommend that patients protect themselves from UV radiation using sunscreen. Five sunscreen ingredients have been shown to protect mice against skin tumors (see [[sunscreen]]). |
|||
[edit] Animal skin products |
|||
The term skin refers to the covering of a small animal, such as a sheep, goat (goatskin), pig, snake (snakeskin) etc or the young of a large animal. |
|||
However, some sunscreen chemicals produce potentially harmful substances if they are illuminated while in contact with living cells.<ref name=Parsons> {{cite journal |author=Xu, C.; Green, Adele; Parisi, Alfio; Parsons, Peter G |year= 2001 |month= |title= Photosensitization of the Sunscreen Octyl p-Dimethylaminobenzoate b UVA in Human Melanocytes but not in Keratinocytes. |journal= Photochemistry and Photobiology |volume= 73 |issue= 6 |pages=600–604 |id= |url=|doi= 10.1562/0031-8655(2001)073<0600:POTSOP>2.0.CO;2}}</ref><ref name=Knowland1993>{{cite journal |author=Knowland, John; McKenzie, Edward A.; McHugh, Peter J.; Cridland, Nigel A. |title= Sunlight-induced mutagenicity of a common sunscreen ingredient. | journal= FEBS Letters |volume= 324(3) |pages=309–313 |year=1993 |doi= 10.1016/0014-5793(93)80141-G }}</ref><ref name=Damiani1999> {{cite journal |author=Damiani, E.; Greci, L.; Parsons, R.; Knowland |title= Nitroxide radicals protect DNA from damage when illuminated in vitro in the presence of dibenzoylmethane and a common sunscreen ingredient. |journal= Free Radic. Biol. Med. |volume= 26 |issue= |pages=809–816 |year=1999 |doi= 10.1016/S0891-5849(98)00292-5 }}</ref> The amount of sunscreen which penetrates through the [[stratum corneum]] may or may not be large enough to cause damage. In one study of sunscreens, the authors write:<ref>Chatelaine, E.; Gabard, B.; Surber, C. (2003) ''[http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowPDF&ArtikelNr=68291&ProduktNr=224219&Ausgabe=228903&filename=68291.pdf pdf Skin Penetration and Sun Protection Factor of Five UV Filters: Effect of the Vehicle]'', Skin Pharmacol. Appl. Skin Physiol., 16:28-35 DOI: 10.1159/000068291</ref> |
|||
The term hides or rawhide refers to the covering of a large adult animal such as a cow, buffalo, horse etc. |
|||
<blockquote>The question whether UV filters acts on or in the skin has so far not been fully answered. Despite the fact that an answer would be a key to improve formulations of sun protection products, many publications carefully avoid addressing this question.</blockquote> |
|||
Skins and hides from different animals are used for clothing, bags and other consumer products, usually in the form of leather, but also furs. |
|||
In an experiment by Hanson et al. that was published in 2006, the amount of harmful [[reactive oxygen species]] (ROS) was measured in untreated and in sunscreen treated skin. In the first 20 minutes the film of sunscreen had a protective effect and the number of ROS species was smaller. After 60 minutes, however, the amount of absorbed sunscreen was so high, that the amount of ROS was higher in the sunscreen treated skin than in the untreated skin.<ref name="Hanson"/> |
|||
Skin can also be cooked to make Pork Rind or Cracklin. The skin on roasted chicken and turkey is another coveted delicacy. |
|||
==== Eye ==== |
|||
High intensities of UVB light are hazardous to the eyes, and exposure can cause ''[[welder's flash]]'' ([[photokeratitis]] or [[arc eye]]) and may lead to [[cataract]]s, [[pterygium]],<ref>{{cite paper | author = Nolan, T. M. et al. | title = The Role of Ultraviolet Irradiation and Heparin-Binding Epidermal Growth Factor-Like Growth Factor in the Pathogenesis of Pterygium |date=2003 | publisher = American Journal of Pathology | url = http://ajp.amjpathol.org/cgi/content/abstract/162/2/567}}</ref><ref>{{cite journal | author = Di Girolamo, N. et al. | title = Epidermal Growth Factor Receptor Signaling Is Partially Responsible for the Increased Matrix Metalloproteinase-1 Expression in Ocular Epithelial Cells after UVB Radiation |date=2005 | journal = American Journal of Pathology | volume = 167 | issue = 2| pages = 489–503 | url = http://ajp.amjpathol.org/cgi/content/abstract/167/2/489 | pmid = 16049334 | month = Aug | day = 01}}</ref> and [[pinguecula]] formation. |
|||
UV light is absorbed by molecules known as [[chromophore]]s, which are present in the eye cells and tissues. Chromophores absorb light energy from the various wavelengths at different rates - a pattern known as [[absorption spectrum]]. If too much UV light is absorbed, eye structures such as the [[cornea]], the [[Lens (anatomy)|lens]] and the [[retina]] can be damaged.<ref>{{cite web |
|||
[edit] Skin layers |
|||
| title = The "Burning" Facts of UV Light |
|||
Skin is composed of three primary layers: |
|||
| publisher = [[SunGlassesUK.com]] |
|||
| url = http://www.sunglassesuk.com/pr1/press_release/the_burning_facts_of_uv_light.asp |
|||
| accessdate = 2009-04-16}}</ref> |
|||
[[Protective eyewear]] is beneficial to those who are working with or those who might be exposed to ultraviolet radiation, particularly short wave UV. Given that light may reach the eye from the sides, full coverage eye protection is usually warranted if there is an increased risk of exposure, as in high altitude mountaineering. Mountaineers are exposed to higher than ordinary levels of UV radiation, both because there is less atmospheric filtering and because of reflection from snow and ice. |
|||
the epidermis, which provides waterproofing and serves as a barrier to infection; |
|||
the dermis, which serves as a location for the appendages of skin; and |
|||
the hypodermis (subcutaneous adipose layer). |
|||
Ordinary, untreated [[eyeglasses]] give some protection. Most plastic lenses give more protection than glass lenses, because, as noted above, glass is transparent to UVA and the common acrylic plastic used for lenses is less so. Some plastic lens materials, such as [[polycarbonate]], inherently block most UV. There are protective treatments available for eyeglass lenses that need it which will give better protection. But even a treatment that ''completely'' blocks UV will not protect the eye from light that arrives around the lens. |
|||
[edit] Epidermis |
|||
Epidermis, "epi" coming from the Greek meaning "over" or "upon", is the outermost layer of the skin. It forms the waterproof, protective wrap over the body's surface and is made up of stratified squamous epithelium with an underlying basal lamina. |
|||
== Degradation of polymers, pigments and dyes == |
|||
The epidermis contains no blood vessels, and cells in the deepest layers are nourished by diffusion from blood capillaries extending to the upper layers of the dermis. The main type of cells which make up the epidermis are Merkel cells, keratinocytes, with melanocytes and Langerhans cells also present. The epidermis can be further subdivided into the following strata (beginning with the outermost layer): corneum, lucidum (only in palms of hands and bottoms of feet), granulosum, spinosum, basale. Cells are formed through mitosis at the basale layer. The daughter cells (see cell division) move up the strata changing shape and composition as they die due to isolation from their blood source. The cytoplasm is released and the protein keratin is inserted. They eventually reach the corneum and slough off (desquamation). This process is called keratinization and takes place within about 27 days. This keratinized layer of skin is responsible for keeping water in the body and keeping other harmful chemicals and pathogens out, making skin a natural barrier to infection. |
|||
[[Image:Failedrope1.jpg|thumb|left|160px|UV damaged [[polypropylene]] rope (left) and new rope (right)]] |
|||
Many [[polymers]] used in consumer products are degraded by UV light, and need addition of UV absorbers to inhibit attack, especially if the products are used outdoors and so exposed to [[sunlight]]. The problem appears as discoloration or fading, [[cracking]] and sometimes, total product disintegration if cracking has proceeded far enough. The rate of attack increases with exposure time and sunlight intensity. |
|||
It is known as [[UV degradation]], and is one form of [[polymer degradation]]. Sensitive polymers include thermoplastics, such as [[polypropylene]] and [[polyethylene]] as well as speciality fibres like [[aramid]]s. UV absorption leads to chain degradation and loss of strength at sensitive points in the chain structure. They include [[tertiary carbon]] atoms, which in [[polypropylene]] occur in every [[repeat unit]]. Aramid rope must be shielded by covering it with a sheath of thermoplastic if it is to retain its strength. |
|||
[also see: image rotating (1.1 mb) ] |
|||
Optical Coherence Tomography tomogram of fingertip, depicting stratum corneum (~500 µm thick) with stratum disjunctum on top and stratum lucidum (connection to stratum spinosum) in the middle. At the bottom superficial parts of the dermis. Sweatducts are clearly visible. |
|||
[edit] Components |
|||
The epidermis contains no blood vessels, and is nourished by diffusion from the dermis. The main type of cells which make up the epidermis are keratinocytes, melanocytes, Langerhans cells and Merkels cells. The epidermis helps the skin to regulate body temperature.[citation needed] |
|||
[[Image:Irspec2.jpg|thumb|right|160px|IR spectrum showing carbonyl absorption due to UV degradation of [[polyethylene]]]] |
|||
In addition, many [[pigments]] and [[dyes]] absorb UV and change colour, so [[paintings]] and [[textiles]] may need extra protection both from sunlight and fluorescent bulbs, two common sources of UV radiation. Old and [[antique]] paintings such as [[watercolour painting]]s for example, usually need to be placed away from direct sunlight. Common window [[glass]] provides some protection by absorbing some of the harmful UV, but valuable artifacts need extra shielding. Many museums place black curtains over [[watercolour painting]]s and ancient textiles, for example. Since watercolours can have very low pigment levels, they need extra protection from the effects of UV light. |
|||
[edit] Layers |
|||
Epidermis is divided into several layers where cells are formed through mitosis at the innermost layers. They move up the strata changing shape and composition as they differentiate and become filled with keratin. They eventually reach the top layer called stratum corneum and are sloughed off, or desquamated. This process is called keratinization and takes place within weeks. The outermost layer of the epidermis consists of 25 to 30 layers of dead cells. |
|||
== Blockers and absorbers == |
|||
Ultraviolet Light Absorbers (UVAs) are molecules used in organic materials ([[polymers]], [[paints]], etc.) to absorb UV light in order to reduce the [[UV degradation]] (photo-oxidation) of a material. A number of different UVAs exist with different absorption properties. UVAs can disappear over time, so monitoring of UVA levels in weathered materials is necessary. |
|||
In [[sunscreen]], ingredients which absorb UVA/UVB rays, such as avobenzone and octyl methoxycinnamate, are known as absorbers. They are contrasted with physical "blockers" of UV radiation such as [[titanium dioxide]] and [[zinc oxide]]. (See [[sunscreen]] for a more complete list.) |
|||
[edit] Sublayers |
|||
Epidermis is divided into the following 5 sublayers or strata: |
|||
== Applications of UV == |
|||
Stratum corneum |
|||
=== Security === |
|||
Stratum lucidum |
|||
[[Image:RBC Visa UV.jpg|right|thumb|A [[bird]] appears on many Visa [[credit card]]s when held under a UV light source]] |
|||
Stratum granulosum |
|||
To help thwart [[counterfeiting|counterfeiters]], sensitive documents (e.g. [[credit card]]s, [[driver's license]]s, [[passport]]s) may also include a UV [[watermark]] that can only be seen when viewed under a UV-emitting light. Passports issued by most countries usually contain UV sensitive inks and security threads. [[Visa (document)|Visa]] stamps and stickers on passports of visitors contain large and detailed seals invisible to the [[naked eye]] under normal lights, but strongly visible under UV illumination. Passports issued by many nations have UV sensitive watermarks on all pages of the passport. Currencies of various countries' [[Banknotes of the Canadian dollar|banknotes]] have an image, as well as many multicolored fibers, that are visible only under ultraviolet light. |
|||
Stratum spinosum |
|||
Stratum germinativum (also called "stratum basale") |
|||
Mnemonics that are good for remembering the layers of the skin (using "stratum basale" instead of "stratum germinativum"): |
|||
Some brands of [[pepper spray]] will leave an invisible chemical (UV Dye) that is not easily washed off on a pepper sprayed attacker, which would help police identify them later.<ref>{{cite web | title = Pepper Spray FAQ | url = http://www.worthprotectionsecurity.com/how-to-use-pepper-spray.htm}}</ref> |
|||
"Cher Likes Getting Skin Botoxed" (from superficial to deep) |
|||
"Before Signing, Get Legal Counsel" (from deep to superficial) |
|||
Blood capillaries are found beneath the epidermis, and are linked to an arteriole and a venule. Arterial shunt vessels may bypass the network in ears, the nose and fingertips. |
|||
=== Forensics === |
|||
Dermis |
|||
UV is an investigative tool at the crime scene helpful in locating and identifying bodily fluids (semen, blood, bile etc.) |
|||
The distribution of the bloodvessels in the skin of the sole of the foot. (Corium - TA alternate term for dermis - is labeled at upper right.) |
|||
A diagrammatic sectional view of the skin (click on image to magnify). (Dermis labeled at center right.) |
|||
Gray's subject #234 1065 |
|||
MeSH Dermis |
|||
Dorlands/Elsevier Skin |
|||
=== Fluorescent lamps === |
|||
[edit] Dermis |
|||
[[Fluorescent lamp]]s produce UV radiation by ionising low-pressure [[mercury (element)|mercury]] vapour. A phosphorescent coating on the inside of the tubes absorbs the UV and converts it to visible light. |
|||
The dermis is the layer of skin beneath the epidermis that consists of connective tissue and cushions the body from stress and strain. The dermis is tightly connected to the epidermis by a basement membrane. It also harbors many Mechanoreceptor/nerve endings that provide the sense of touch and heat. It contains the hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels and blood vessels. The blood vessels in the dermis provide nourishment and waste removal to its own cells as well as the Stratum basale of the epidermis. |
|||
The main mercury emission wavelength is in the UVC range. Unshielded exposure of the skin or eyes to mercury arc lamps that do not have a conversion phosphor is quite dangerous. |
|||
The dermis is structurally divided into two areas: a superficial area adjacent to the epidermis, called the papillary region, and a deep thicker area known as the reticular region. |
|||
The light from a mercury lamp is predominantly at discrete wavelengths. Other practical UV sources with more continuous emission spectra include [[Xenon flash lamp|xenon arc lamps]] (commonly used as sunlight simulators), deuterium arc lamps, [[mercury-xenon arc lamp]]s, metal-halide arc lamps, and tungsten-halogen incandescent lamps. |
|||
=== Astronomy === |
|||
[edit] Papillary region |
|||
[[Image:Satellite Footprints Seen in Jupiter Aurora.jpg|thumb|right|[[Aurora (phenomenon)|Aurora]] at [[Jupiter]]'s north pole as seen in ultraviolet light by the [[Hubble Space Telescope]].]] |
|||
The papillary region is composed of loose areolar connective tissue. It is named for its fingerlike projections called papillae, that extend toward the epidermis. The papillae provide the dermis with a "bumpy" surface that interdigitates with the epidermis, strengthening the connection between the two layers of skin. |
|||
In [[astronomy]], very hot objects preferentially emit UV radiation (see [[Wien's law]]). Because the ozone layer blocks many UV frequencies from reaching telescopes on the surface of the Earth, most UV observations are made from space. (See ''[[UV astronomy]]'', ''[[space observatory]]''.) |
|||
In the palms, fingers, soles, and toes, the influence of the papillae projecting into the epidermis forms contours in the skin's surface. These are called friction ridges, because they help the hand or foot to grasp by increasing friction. Friction ridges occur in patterns (see: fingerprint) that are genetically and epigenetically determined and are therefore unique to the individual, making it possible to use fingerprints or footprints as a means of identification. |
|||
=== Hunting === |
|||
Hunters can use UV lights to follow the blood trail of a wounded deer, elk or other game animal. |
|||
=== Biological surveys and pest control === |
|||
[edit] Reticular region |
|||
Some animals, including birds, reptiles, and insects such as bees, can see into the near ultraviolet. Many fruits, flowers, and seeds stand out more strongly from the background in ultraviolet wavelengths as compared to human color vision. Scorpions glow or take on a yellow to green color under UV illumination thus assisting in the control of these sometimes fatally venomous arachnids. Many birds have patterns in their plumage that are invisible at usual wavelengths but observable in ultraviolet, and the urine and other secretions of some animals, including dogs, cats, and human beings, is much easier to spot with ultraviolet. Urine trails of rodents can be detected by pest control technicians for proper treatment of infested dwellings. |
|||
The reticular region lies deep in the papillary region and is usually much thicker. It is composed of dense irregular connective tissue, and receives its name from the dense concentration of collagenous, elastic, and reticular fibers that weave throughout it. These protein fibers give the dermis its properties of strength, extensibility, and elasticity. |
|||
Many insects use the ultraviolet wavelength emissions from celestial objects as references for flight navigation. A local ultraviolet emissor will normally disrupt the navigation process and will eventually attract the flying insect. |
|||
Also located within the reticular region are the roots of the hair, sebaceous glands, sweat glands, receptors, nails, and blood vessels. |
|||
[[Image:ultraviolet trap entomologist.jpg|thumb|right|Entomologist using a UV light for collecting [[beetles]] in the [[Paraguay]]an [[Chaco]].]] |
|||
Ultraviolet traps called [[bug zapper]]s are used to eliminate various small flying insects. They are attracted to the UV light, and are killed using an electric shock, or trapped once they come into contact with the device. Different designs of ultraviolet light traps are also used by [[entomologists]] for [[collect]]ing [[nocturnal]] insects during [[faunistic]] survey studies. |
|||
=== Spectrophotometry === |
|||
Tattoo ink is held in the dermis. Stretch marks from pregnancy are also located in the dermis. |
|||
[[UV/VIS spectroscopy]] is widely used as a technique in [[chemistry]], to analyze [[chemical structure]], most notably [[conjugated system]]s. UV radiation is often used in visible [[spectrophotometry]] to determine the existence of fluorescence in a given sample. |
|||
=== Sanitary compliance === |
|||
UV lamps including newer LEDs (light emitting diode) aid in the detection of organic mineral deposits that remain on surfaces where periodic cleaning and sanitizing may not be properly accomplished. Both urine and phosphate soaps are easily detected using UV inspection. Pet urine deposits in carpeting or other hard surfaces can be detected for accurate treatment and removal of mineral tracers and the odor causing bacteria that feeds on proteins within. Many hotel and hospitality industries use UV lamp to inspect for unsanitary bedding to determine life cycle for mattress restoration as well as general performance of the cleaning staff. |
|||
=== Air purification === |
|||
[edit] Hypodermis |
|||
Using a catalytic reaction from titanium dioxide and UV light exposure, a strong oxidative effect occurs on any organic that passes through the media converting otherwise irritating pathogens, pollens, and mold spores into harmless inert byproducts. |
|||
The hypodermis is not part of the skin, and lies below the dermis. Its purpose is to attach the skin to underlying bone and muscle as well as supplying it with blood vessels and nerves. It consists of loose connective tissue and elastin. The main cell types are fibroblasts, macrophages and adipocytes (the hypodermis contains 50% of body fat). Fat serves as padding and insulation for the body. |
|||
=== Analyzing minerals === |
|||
Microorganisms like Staphylococcus epidermidis colonize the skin surface. The density of skin flora depends on region of the skin. The disinfected skin surface gets recolonized from bacteria residing in the deeper areas of the hair follicle, gut and urogenital openings. |
|||
[[Image:Fluorescent minerals hg.jpg|thumb|right|A collection of [[mineral]] samples brilliantly fluorescing at various wavelengths as seen while being irradiated by UV light.]] |
|||
Ultraviolet lamps are also used in analyzing [[minerals]], [[gemstone|gems]], and in other detective work including authentication of various [[collectible]]s. Materials may look the same under visible light, but [[fluorescence|fluoresce]] to different degrees under ultraviolet light; or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet. |
|||
=== Authentication === |
|||
[edit] See also |
|||
In other detective work including authentication of various collectibles art, and . Detecting counterfeit currency absent of marker dyes. Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light; or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet. |
|||
Look up skin in Wiktionary, the free dictionary. |
|||
Acid mantle |
|||
Anthropodermic bibliopegy |
|||
Artificial skin |
|||
Callus - thick area of skin |
|||
Cosmetics and cosmetic surgery |
|||
Cutaneous structure development |
|||
Dermatology - branch of medicine |
|||
Fingerprint - skin on fingertips |
|||
Hair - including hair follicles in skin |
|||
Skin color |
|||
Hyperpigmentation - about excess skin color |
|||
Intertriginous |
|||
Moult |
|||
Meissner's corpuscle |
|||
Nails - fingernails or toenails |
|||
Pacinian corpuscle |
|||
Polyphenol antioxidant |
|||
Skin disease |
|||
Skin diseases, list of |
|||
Skin lesion |
|||
Skin repair |
|||
Sweat - description of perspiration |
|||
Superficial fascia |
|||
=== Chemical markers === |
|||
[edit] References |
|||
UV fluorescent [[dye]]s are used in many applications (for example, [[biochemistry]] and [[Forensic science|forensics]]). The [[Green Fluorescent Protein]] (GFP) is often used in [[genetics]] as a marker. Many substances, such as proteins, have significant light absorption bands in the ultraviolet that are of use and interest in biochemistry and related fields. UV-capable spectrophotometers are common in such laboratories. |
|||
^ a b c d e f g h i j k l "Skin care" (analysis), Health-Cares.net, 2007, webpage: HCcare. |
|||
^ Alibardi L. (2003). Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. J Exp Zoolog B Mol Dev Evol. 298(1):12-41. PMID 12949767 |
|||
^ a b Proksch E, Brandner JM, Jensen JM. (2008).The skin: an indispensable barrier. Exp Dermatol. 17(12):1063-72. PMID 19043850 |
|||
^ a b c Madison KC. (2003). Barrier function of the skin: "la raison d'être" of the epidermis. J Invest Dermatol. 121(2):231-41. PMID 12880413 |
|||
^ a b c Grice EA, Kong HH, Conlan S. (2009). Topographical and Temporal Diversity of the Human Skin Microbiome, Science, 324: 1190 - 1192. doi:10.1126/science.1171700 |
|||
^ a b Pappas S. (2009). Your Body Is a Wonderland ... of Bacteria. ScienceNOW Daily News |
|||
^ Maton, Anthea; Jean Hopkins, Charles William McLaughlin, Susan Johnson, Maryanna Quon Warner, David LaHart, Jill D. Wright (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. ISBN 0-13-981176-1. |
|||
^ Theodor Rosebury. Life on Man: Secker & Warburg, 1969 ISBN 0-670-42793-4 |
|||
^ http://www3.interscience.wiley.com/journal/107640112/abstract |
|||
^ http://www.ingentaconnect.com/content/bsc/ics/2004/00000026/00000002/art00010 |
|||
^ Marks, James G; Miller, Jeffery (2006). Lookingbill and Marks' Principles of Dermatology (4th ed.). Elsevier Inc. ISBN 1-4160-3185-5. |
|||
^ Smith, Wilma and Burns, Catherine. (1999) "Managing the hair and skin of African American pediatric patients." Journal of Pediatric Health Care 13(2):72-8. |
|||
^ Weller, Richard; John Hunter, John Savin, Mark Dahl (2008). Clinical Dermatology (4th ed.). Malden, Massachusetts, USA: Blackwell Publishing. pp. 268. ISBN 978-1-4051-4663-0. |
|||
^ NIH Human Microbiome Project. |
|||
[show]v • d • eIntegumentary system |
|||
Skin Epidermis Stratum corneum · Stratum lucidum · Stratum granulosum · Stratum spinosum · Stratum basale |
|||
Basement membrane zone Basal keratinocyte · Lamina lucida · Lamina densa |
|||
Dermis Papillary · Reticular |
|||
Subcutaneous tissue Panniculus adiposus · Panniculus carnosus |
|||
Adnexa Glands Sweat glands: Apocrine · Eccrine |
|||
Sebaceous |
|||
Hair Outer root sheath |
|||
Inner root sheath |
|||
Shaft Cuticle · Cortex · Medulla · Bulb with matrix cells · Hair papilla |
|||
Muscle Arrector pili |
|||
Nail Matrix · Lunula · Nail plate · Eponychium · Paronychium · Hyponychium |
|||
Other Skin flora |
|||
[show]v • d • eHuman anatomical features |
|||
Head Skull · Forehead · Eye · Ear · Nose · Mouth · Tongue · Teeth · Jaw · Face · Cheek · Chin |
|||
Neck Throat · Adam's apple |
|||
Torso Shoulder · Spine · Breast (Tail of Spence · Nipple) · Chest · Ribcage · Abdomen · Navel |
|||
Sex organs (Clitoris · Vagina · Penis · Scrotum · Testicle) – Hip · Anus · Buttocks |
|||
Limbs Upper limb Arm · Elbow · Forearm · Wrist · Hand · Finger (Thumb · Index · Middle · Ring · Little) · |
|||
Lower limb Leg · Lap · Thigh · Knee · Calf · Heel · Ankle · Foot · Toe (Hallux · Fifth) |
|||
Skin Hair |
|||
=== Photochemotherapy === |
|||
Retrieved from "http://en.wikipedia.org/wiki/Skin" |
|||
Exposure to UVA light while the skin is hyper-photosensitive by taking [[psoralen]]s is an effective treatment for [[psoriasis]] called [[PUVA]]. Due to [[psoralens]] potentially causing damage to the [[liver]], [[PUVA]] may only be used a limited number of times over a patient's lifetime |
|||
Categories: Skin | Organs |
|||
Hidden categories: Articles needing additional references from January 2008 | Articles to be merged from April 2009 | All articles to be merged | All articles with unsourced statements | Articles with unsourced statements from November 2008ViewsArticle Discussion Edit this page History Personal toolsLog in / create account Navigation |
|||
Main page |
|||
Contents |
|||
Featured content |
|||
Current events |
|||
Random article |
|||
Search |
|||
Interaction |
|||
About Wikipedia |
|||
Community portal |
|||
Recent changes |
|||
Contact Wikipedia |
|||
Donate to Wikipedia |
|||
Help |
|||
Toolbox |
|||
What links here |
|||
Related changes |
|||
Upload file |
|||
Special pages |
|||
Printable version |
|||
Permanent link |
|||
Cite this page |
|||
Languages |
|||
Afrikaans |
|||
العربية |
|||
Aymar aru |
|||
Brezhoneg |
|||
Български |
|||
Català |
|||
Česky |
|||
Cymraeg |
|||
Dansk |
|||
Deutsch |
|||
ދިވެހިބަސް |
|||
Eesti |
|||
Ελληνικά |
|||
Español |
|||
Esperanto |
|||
فارسی |
|||
Français |
|||
Gàidhlig |
|||
Galego |
|||
Хальмг |
|||
한국어 |
|||
Hrvatski |
|||
Ido |
|||
Bahasa Indonesia |
|||
Íslenska |
|||
Italiano |
|||
עברית |
|||
Basa Jawa |
|||
Kapampangan |
|||
ქართული |
|||
Latina |
|||
Latviešu |
|||
Lietuvių |
|||
Lingála |
|||
Magyar |
|||
Македонски |
|||
मराठी |
|||
Bahasa Melayu |
|||
Nederlands |
|||
日本語 |
|||
Norsk (bokmål) |
|||
Norsk (nynorsk) |
|||
Nouormand |
|||
Pangasinan |
|||
Polski |
|||
Português |
|||
Română |
|||
Runa Simi |
|||
Русский |
|||
Саха тыла |
|||
Sicilianu |
|||
Simple English |
|||
Slovenčina |
|||
Slovenščina |
|||
Српски / Srpski |
|||
Basa Sunda |
|||
Suomi |
|||
Svenska |
|||
Tagalog |
|||
தமிழ் |
|||
తెలుగు |
|||
ไทย |
|||
Türkçe |
|||
Українська |
|||
اردو |
|||
Tiếng Việt |
|||
Võro |
|||
ייִדיש |
|||
Žemaitėška |
|||
中文 |
|||
=== Phototherapy === |
|||
This page was last modified on 6 June 2009 at 03:36. All text is available under the terms of the GNU Free Documentation License. (See Copyrights for details.) |
|||
{{Refimprove|date=April 2007}} |
|||
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a U.S. registered 501(c)(3) tax-deductible nonprofit charity. |
|||
{{Expert-subject|health|date=March 2008}} |
|||
Privacy policy About Wikipedia Disclaimers |
|||
Exposure to UVB light, particularly the 310 nm narrowband UVB range, is an effective long-term treatment for many skin conditions like [[psoriasis]], [[vitiligo]], [[eczema]], and others.<ref>[http://nobelprize.org/nobel_prizes/medicine/laureates/1903/press.html UV is used to treat tuberculosis of the skin]</ref> UVB phototherapy does not require additional medications or topical preparations for the therapeutic benefit; only the light exposure is needed. However, phototherapy can be effective when used in conjunction with certain topical treatments such as anthralin, coal tar, and Vitamin A and D derivatives, or systemic treatments such as methotrexate and soriatane.<ref> |
|||
{{cite web |
|||
|title = UVB Phototherapy |
|||
|url = http://www.psoriasis.org/treatment/psoriasis/phototherapy/uvb.php |
|||
|archiveurl=http://web.archive.org/web/20070622180124/http://www.psoriasis.org/treatment/psoriasis/phototherapy/uvb.php |
|||
|archivedate=2007-06-22 |
|||
|format=php |
|||
|accessdate=2007-09-23 |
|||
|publisher=National Psoriasis Foundation, USA}}</ref> |
|||
Typical treatment regimes involve short exposure to UVB rays 3 to 5 times a week at a hospital or clinic, and up to 30 or more sessions may be required before results are noticeable. Almost all of the conditions that respond to UVB light are chronic problems, so continual treatment is required to keep those problems in check. Home UVB systems are common solutions for those whose conditions respond to treatment. Home systems permit the patient to treat themselves every other day (the ideal treatment regimen for most) without the frequent, costly trips to the office/clinic and back. |
|||
Side effects may include itching and redness of the skin due to UVB exposure, and possibly sunburn, if patients do not minimize exposure to natural UV rays during treatment days. Cataracts can frequently develop if the eyes are not protected from UVB light exposure. There is no link between an increase in the patient's risk for skin cancer and the proper use of UVB phototherapy. "Proper use" is generally defined as reaching the "Sub-Erythemic Dose" (S.E.D.), the maximum amount of UVB your skin can receive ''without'' burning. |
|||
Certain fungal growths under the toenail can be treated using a specific wavelength of UV delivered from a high power LED (light emitting diode) and can be safer than traditional systemic drugs. |
|||
=== Photolithography === |
|||
Ultraviolet radiation is used for very fine resolution [[photolithography]], a procedure where a chemical known as a photoresist is exposed to UV radiation which has passed through a mask. The light allows chemical reactions to take place in the photoresist, and after development (a step that either removes the exposed or unexposed photoresist), a geometric pattern which is determined by the mask remains on the sample. Further steps may then be taken to "etch" away parts of the sample with no photoresist remaining. |
|||
UV radiation is used extensively in the electronics industry because photolithography is used in the manufacture of [[semiconductor]]s, [[integrated circuit]] components<ref>{{cite web | title = Deep UV Photoresists | url = http://www.almaden.ibm.com/st/chemistry/lithography/deep_uv/}}</ref> and [[printed circuit board]]s. |
|||
=== Checking electrical insulation === |
|||
A new application of UV is to detect [[corona discharge]] (often simply called "corona") on electrical apparatus. Degradation of insulation of electrical apparatus or pollution causes corona, wherein a strong electric field ionizes the air and excites nitrogen molecules, causing the emission of ultraviolet radiation. The corona degrades the insulation level of the apparatus. Corona produces [[ozone]] and to a lesser extent [[nitrogen oxide]] which may subsequently react with water in the air to form [[nitrous acid]] and [[nitric acid]] vapour in the surrounding air.<ref>{{cite web | title = Corona - The Daytime UV Inspection Magazine | url = http://www.seeing-corona.com/}}</ref> |
|||
=== Sterilization === |
|||
{{main|Ultraviolet germicidal irradiation}} |
|||
[[Image:UV-ontsmetting laminaire-vloeikast.JPG|thumb|right|A low pressure mercury vapor discharge tube floods the inside of a [[Fume hood|hood]] with shortwave UV light when not in use, [[sterilize|sterilizing]] microbiological contaminants from irradiated surfaces.]] |
|||
Ultraviolet lamps are used to [[sterilization (microbiology)|sterilize]] workspaces and tools used in biology laboratories and medical facilities. Commercially-available low pressure [[mercury-vapor lamps]] emit about 86% of their light at 254 nanometers (nm) which coincides very well with one of the two peaks of the germicidal effectiveness curve (i.e., effectiveness for UV absorption by DNA). One of these peaks is at about 265 nm and the other is at about 185 nm. Although 185 nm is better absorbed by DNA, the [[quartz glass]] used in commercially-available lamps, as well as environmental media such as water, are more opaque to 185 nm than 254 nm (C. von Sonntag et al., 1992). UV light at these germicidal wavelengths causes adjacent [[thymine]] molecules on DNA to [[dimer]]ize, if enough of these defects accumulate on a microorganism's DNA its replication is inhibited, thereby rendering it harmless (even though the organism may not be killed outright). However, since microorganisms can be shielded from ultraviolet light in small cracks and other shaded areas, these lamps are used only as a supplement to other sterilization techniques. |
|||
=== Disinfecting drinking water === |
|||
UV radiation can be an effective [[viricide]] and [[bactericide]]. Disinfection using UV radiation is commonly used in wastewater treatment applications and is finding an increased usage in drinking water treatment. Many bottlers of spring water use UV disinfection equipment to sterilize their water. [[Solar water disinfection]] is the process of using [[Polyethylene terephthalate|PET]] bottles and sunlight to disinfect water. |
|||
New York City has approved the construction of a 2 billion gallon per day ultraviolet drinking water disinfection facility.<ref>{{cite paper | title = UV Disinfection for New York City: Bridging Design with Operational Strategies | author = Donna Portoti et al. | publisher = American Water Works Association | format = PDF | url = http://www.cdm.com/NR/rdonlyres/EB81DD91-38EC-48AA-A993-2F08A81917B3/0/StrategiesforNYCUVDinsfectionProject.pdf | accessdate = 2008-12-28}}</ref> There are also several facilities under construction and several in operation that treat waste water with several stages of filters, hydrogen peroxide and UV light to bring the water up to drinking standards. One such facility exists in Orange County, California.<ref>[http://www.latimes.com/news/local/la-me-reclaim2jan02,0,7789563.story?coll=la-home-center Sewage in O.C. goes full circle - Los Angeles Times<!-- Bot generated title -->]</ref><ref>[http://www.wired.com/science/planetearth/multimedia/2008/01/gallery_sewage_plant New Purification Plant Answers California's Water Crisis<!-- Bot generated title -->]</ref> NASA has examined the use of this technology, using titanium dioxide as [[Catalysis|catalyst]], for breaking down harmful products in spacecraft waste water.<ref>{{cite journal|last=Antoniou|first=Maria G.|coauthors=Dionysiou, Dionysios D.|date=30 June 2007|title=Application of immobilized titanium dioxide photocatalysts for the degradation of creatinine and phenol, model organic contaminants found in NASA's spacecrafts wastewater streams|journal=Catalysis Today|publisher=Elsevier|volume=124|issue=3-4|pages=215–223|doi=10.1016/j.cattod.2007.03.054 }}</ref> |
|||
It used to be thought that UV disinfection was more effective for bacteria and viruses, which have more exposed genetic material, than for larger pathogens which have outer coatings or that form cyst states (e.g., [[Giardia]]) that shield their DNA from the UV light. However, it was recently discovered that ultraviolet radiation can be somewhat effective for treating the microorganism [[Cryptosporidium]]. The findings resulted in the use of UV radiation as a viable method to treat drinking |
|||
water. Giardia in turn has been shown to be very susceptible to UV-C when the tests were based on infectivity rather than excystation.<ref>{{cite paper | title = Inactivation of Giardia muris by Low Pressure Ultraviolet Light | author = Ware, M. W. et al. | publisher = United States Environmental Protection Agency | format = PDF | url = http://www.epa.gov/nerl/news/forum2003/water/ware_poster.pdf | accessdate = 2008-12-28}}</ref> It has been found that [[protists]] are able to survive high UV-C doses but are sterilized at low doses. |
|||
[[Solar water disinfection]]<ref>[http://www.sodis.ch/index.htm Solar Water Disinfection<!-- Bot generated title -->]</ref> (SODIS) has been extensively researched in Switzerland and has proven ideal to treat small quantities of water cheaply using natural sunlight. Contaminated water is poured into transparent plastic bottles and exposed to full sunlight for six hours. The sunlight treats the contaminated water through two synergetic mechanisms: UV-A irradiation and increased water temperature. If the water temperatures rises above 50 °C, the disinfection process is three times faster. |
|||
=== Food processing === |
|||
As consumer demand for fresh and "fresh-like" food products increases, the demand for nonthermal methods of [[food processing]] is likewise on the rise. In addition, public awareness regarding the dangers of [[food poisoning]] is also raising demand for improved food processing methods. Ultraviolet radiation is used in several food processes to kill unwanted [[microorganisms]]. UV light can be used to [[pasteurization|pasteurize]] fruit juices by flowing the juice over a high intensity ultraviolet light source.<ref>https://www.rulfsorchard.com/index.php?option=com_rsgallery2&page=inline&id=8&Itemid=34</ref> The effectiveness of such a process depends on the UV [[absorbance]] of the juice (see [[Beer's law]]). |
|||
=== Fire detection === |
|||
Ultraviolet detectors generally use either a solid-state device, such as one based on [[silicon carbide]] or [[aluminium nitride]], or a gas-filled tube as the sensing element. UV detectors which are sensitive to UV light in any part of the spectrum respond to irradiation by [[sunlight]] and [[artificial light]]. A burning hydrogen flame, for instance, radiates strongly in the 185 to 260 nanometer range and only very weakly in the [[IR]] region, while a [[coal]] fire emits very weakly in the UV band yet very strongly at IR wavelengths; thus a fire detector which operates using both UV and IR detectors is more reliable than one with a UV detector alone. Virtually all fires emit some [[thermal radiation|radiation]] in the UVC band, while the [[Sun]]'s radiation at this band is absorbed by the [[Earth's atmosphere]]. The result is that the UV detector is "solar blind", meaning it will not cause an alarm in response to radiation from the Sun, so it can easily be used both indoors and outdoors. |
|||
UV detectors are sensitive to most fires, including [[hydrocarbon]]s, [[metal]]s, [[sulfur]], [[hydrogen]], [[hydrazine]], and [[ammonia]]. [[Arc welding]], electrical arcs, [[lightning]], [[X-ray]]s used in nondestructive metal testing equipment (though this is highly unlikely), and radioactive materials can produce levels that will activate a UV detection system. The presence of UV-absorbing gases and vapors will attenuate the UV radiation from a fire, adversely affecting the ability of the detector to detect flames. Likewise, the presence of an oil mist in the air or an oil film on the detector window will have the same effect. |
|||
=== Herpetology === |
|||
[[Reptile]]s need long wave UV light to metabolize calcium for bone and egg production. Thus, in a typical reptile enclosure, a fluorescent UV lamp is used at one end of the enclosure for calcium absorption and a common incandescent bulb is used at the other end for heat (basking). |
|||
=== Curing of electronic potting resins === |
|||
Electronic components that require clear transparency for light to exit or enter (photo voltaic panels and sensors)can be potted using acrylic resins that are cured using UV light energy. The advantages are low VOC emissions and rapid curing. |
|||
=== Curing of inks, adhesives, varnishes and coatings === |
|||
Certain inks, coatings and [[adhesives]] are formulated with photoinitiators and resins. When exposed to the correct energy and irradiance in the required band of UV light, polymerization occurs, and so the adhesives harden or cure. Usually, this reaction is very quick, a matter of a few seconds. Applications include glass and plastic bonding, [[optical fiber]] coatings, the coating of flooring, [[UV Coating]] and paper finishes in offset [[printing]], and dental fillings. Curing of decorative finger nail "gels". Industrial UV curing systems can be found at DDU Enterprises, Inc.'s website "Doctoruv.com" |
|||
An industry has developed around the manufacture of UV sources for UV curing applications. This includes [[UV lamps]], UV [[LED]]s and [[Excimer]] Flash lamps. Fast processes such as flexo or offset printing require high intensity light focused via reflectors onto a moving substrate and medium and high pressure [[Mercury (element)|Hg]] (mercury) or [[Fe]] (iron, doped) based bulbs are used which can be energised with electric arc or microwaves. Currently, LED-based sources (focused and not focused) are achieving comparable power levels and are expected to become as important as Hg lamps in these applications.{{Fact|date=March 2009}} Lower power sources (fluorescent lamps, LED) can be used for static applications and in some cases, small high pressure lamps can have light focused and transmitted to the work area via liquid filled or fibre optic light guides. |
|||
=== Deterring substance abuse in public places === |
|||
{{Refimprove|section|date=April 2007}} |
|||
UV lights have been installed in some parts of the world in public restrooms, and on public transport, for the purpose of deterring substance abuse. The blue color of these lights, combined with the fluorescence of the skin, make it harder for intravenous [[drug user]]s to find a vein.<ref>[http://iccoventry.icnetwork.co.uk/0100news/0150swarksnews/tm_objectid=14644486&method=full&siteid=50003&headline=public-toilets--lighting-has-wrong-effect-name_page.html Public toilets' lighting has wrong effect] from Coventry Telegraph</ref> The efficacy of these lights for that purpose has been questioned, with some suggesting that drug users simply find a vein outside the public restroom and mark the spot with a marker for accessibility when inside the restroom. There is currently no published evidence supporting the idea of a deterrent effect. |
|||
=== Sun tanning === |
|||
[[Sun tanning]] describes a darkening of the skin in a natural physiological response stimulated by exposure to [[ultraviolet radiation]] from [[sunlight|sunshine]] (or a [[sunbed]]). With excess exposure to the sun, a suntanned area can also develop sunburn. The increased production of melanin is triggered by the [[direct DNA damage]].<ref name=Agar2005> {{cite journal |author=Nita Agar; Antony R. Young |title=Review: Melanogenesis: a photoprotective response to DNA damage? |journal=Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis |volume=571 |issue=1-2 |pages=121–132 |year=2005 |pmid=10.1016/j.mrfmmm.2004.11.016 |url=http://www.ncbi.nlm.nih.gov/pubmed/15748643 |doi=10.1016/j.mrfmmm.2004.11.016}}</ref> This kind of damage is recognized by the body and as a defense against UV radiation the skin produces more melanin. Melanin dissipates the UV energy as harmless heat, and therefore it is an excellent [[photoprotection|photoprotectant]]. Melanin protects against the direct DNA damage and against the [[indirect DNA damage]]. Sunscreen protects only against the direct DNA damage, but increases the indirect DNA damage<ref name=Parsons> {{cite journal |author=Xu, C.; Green, Adele; Parisi, Alfio; Parsons, Peter G |year= 2001 |month= |title= Photosensitization of the Sunscreen Octyl p-Dimethylaminobenzoate b UVA in Human Melanocytes but not in Keratinocytes. |journal= Photochemistry and Photobiology |volume= 73 |issue= 6 |pages=600–604 |id= |url=|doi= 10.1562/0031-8655(2001)073<0600:POTSOP>2.0.CO;2}}</ref><ref name="Knowland1993"/><ref name="Damiani1999"/> - this causes the higher amount of melanoma that had been found repeatedly in sunscreen users compared to non-users.<ref name=Garland> {{cite journal |author=Garland C, Garland F, Gorham E |title=Could sunscreens increase melanoma risk? |url= http://www.ajph.org/cgi/reprint/82/4/614 |journal=Am J Public Health |volume=82 |issue=4 |pages=614–5 |year=1992 |pmid=1546792 |doi=10.2105/AJPH.82.4.614}}</ref><ref name=Westerdahl2000> {{cite journal |author=Westerdahl J; Ingvar C; Masback A; Olsson H |title= Sunscreen use and malignant melanoma. | journal= International journal of cancer. Journal international du cancer |volume=87 |pages=145–50 |year=2000 |doi= 10.1002/1097-0215(20000701)87:1<145::AID-IJC22>3.0.CO;2-3 }}</ref><ref name=Autier> {{cite journal |author=Autier P; Dore J F; Schifflers E; et al. |title=Melanoma and use of sunscreens: An EORTC case control study in Germany, Belgium and France |url= |journal=Int. J. Cancer |volume=61 |issue= |pages=749–755 |year=1995 |doi=10.1002/ijc.2910610602}}</ref><ref name=Weinstock> {{cite journal |author=Weinstock, M. A. |title=Do sunscreens increase or decrease melanoma risk: An epidemiologic evaluation. |url= |journal=Journal of Investigative Dermatology Symposium Proceedings |volume=4 |issue= |pages= 97–100 |year=1999 |doi=10.1038/sj.jidsp.}}</ref><ref name=Vainio> {{cite journal |author=Vainio, H., Bianchini, F. |title=Cancer-preventive effects of sunscreens are uncertain. |url= |journal=Scandinavian Journal of Work Environment and Health |volume=26 |issue= |pages=529–31 |year=2000 |issn=}}</ref> |
|||
=== Erasing EPROM modules === |
|||
Some [[EPROM]] (erasable programmable read-only memory) modules are erased by exposure to UV radiation. These modules often have a transparent glass ([[quartz]]) window on the top of the [[chip]] that allows the UV radiation in. These have been largely superseded by [[EEPROM]] and [[flash memory]] chips in most devices. |
|||
=== Preparing low surface energy polymers === |
|||
UV radiation is useful in preparing low surface energy [[polymer]]s for adhesives. Polymers exposed to UV light will oxidize thus raising the surface energy of the polymer. Once the surface energy of the polymer has been raised, the bond between the adhesive and the polymer will not be smaller. |
|||
=== Reading otherwise illegible papyruses === |
|||
Using multi-spectral imaging it is possible to read illegible [[papyrus]]es, such as the burned papyruses of the [[Villa of the Papyri]] or of [[Oxyrhynchus]], or the [[Archimedes palimpsest]]. The technique involves taking pictures of the illegible papyruses using different filters in the infrared or ultraviolet range, finely tuned to capture certain wavelengths of light. Thus, the optimum spectral portion can be found for distinguishing ink from paper on the papyrus surface. |
|||
=== Lasers === |
|||
Ultraviolet [[lasers]] have applications in industry ([[laser engraving]]), medicine ([[dermatology]] and [[keratectomy]]), [[Free Space Optics|free air secure communications]] and computing ([[optical storage]]). They can be made by applying [[frequency conversion]] to lower-frequency lasers, or from Ce:LiSAF crystals ([[cerium]] [[Dopant|doped]] with lithium strontium aluminum fluoride), a process developed in the 1990s at [[Lawrence Livermore National Laboratory]].<ref>{{cite web |
|||
| last = Marshall |
|||
| first = Chris |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = A simple, reliable ultraviolet laser: the Ce:LiSAF |
|||
| work = |
|||
| publisher = Lawrence Livermore National Laboratory |
|||
| date = 1996 |
|||
| url = https://www.llnl.gov/str/Marshall.html |
|||
| format = |
|||
| doi = |
|||
| accessdate = 2008-01-11}}</ref> |
|||
=== UV solar cells and UV degration of solar cells === |
|||
{{Main|Solar cell|UV degradation}} |
|||
Japan's [[National Institute of Advanced Industrial Science and Technology]] (AIST) has succeeded in developing a [[transparent]] solar cell that uses ultraviolet light to generate electricity but allows visible light to pass through it. Most conventional solar cells use visible and infrared light to generate electricity. In contrast, the innovative new solar cell uses ultraviolet radiation. Used to replace conventional window glass, the installation surface area could be large, leading to potential uses that take advantage of the combined functions of power generation, lighting and temperature control.<ref>http://www.japanfs.org/en/pages/025337.html</ref> |
|||
Also [[PEDOT]]-[[PSS]] solar cells is a ultraviolet (UV) light selective and sensitive photovoltaic cell easily fabricated.<ref>http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=APPLAB000083000011002097000001&idtype=cvips&gifs=yes</ref> |
|||
On the other hand, a [[nanocrystalline]] layer of [[Cu2O]] in the construction of photovoltaic cells increases their ability to utilize UV radiations for photocurrent generation.<ref>[http://www.freepatentsonline.com/6849798.html Photovoltaic cell using stable Cu2O nanocrystals and conductive polymers - Patent 6849798<!-- Bot generated title -->]</ref> |
|||
=== Non-destructive Testing === |
|||
{{Main|Non-destructive_testing}} |
|||
UV light of a specified spectrum and intensity is used to stimulate fluorescent dyes so as to highlight defects in a broad range of materials. These dyes may be carried into surface-breaking defects by capillary action ([[liquid penetrant|liquid penetrant inspection]]) or they may be bound to ferrite particles caught in magnetic leakage fields in ferrous materials ([[magnetic-particle inspection|magnetic particle inspection]]). |
|||
{{Expand-section|date=November 2008}} |
|||
== Evolutionary significance == |
|||
Evolution of early reproductive [[proteins]] and [[enzymes]] is attributed in modern models of [[evolutionary theory]] to ultraviolet light. UVB light causes [[thymine]] base pairs next to each other in genetic sequences to bond together into [[thymine dimers]], a disruption in the strand which reproductive enzymes cannot copy (see picture above). This leads to [[frameshifting]] during genetic replication and [[protein synthesis]], usually killing the organism. As early prokaryotes began to approach the surface of the ancient oceans, before the protective ozone layer had formed, blocking out most wavelengths of UV light, they almost invariably died out. The few that survived had developed enzymes which verified the genetic material and broke up [[thymine dimer]] bonds, known as [[excision repair]] enzymes. Many enzymes and proteins involved in modern [[mitosis]] and [[meiosis]] are similar to excision repair enzymes, and are believed to be evolved modifications of the enzymes originally used to overcome UV light.<ref>{{cite paper |
|||
| author = Margulis, Lynn and Sagan, Dorion |
|||
| title = Origins of Sex: Three Billion Years of Genetic Recombination |
|||
| version = 1 |
|||
| publisher = Yale University Press |
|||
|date=1986 |
|||
| url = http://books.google.com/books?vid=ISBN0300046197&id=3hDVTEk3ioIC&pg=PP1&lpg=PP1&ots=IG3gUOrVBQ&dq=origins+of+sex:+three&sig=kcL3cFKL0NqFQoSXXlsFzQ9mh-8 |
|||
| format = book |
|||
}}</ref> |
|||
== See also == |
|||
* [[Black light]] |
|||
* [[High-energy visible light]] |
|||
* [[Infrared light]] |
|||
* [[Polymer degradation]] |
|||
* [[Sun tanning]] |
|||
* [[Tanning lamp]] |
|||
* [[Titanium dioxide]] |
|||
* [[Ultraviolet photography]] |
|||
* [[UV degradation]] |
|||
* [[UV index]] |
|||
* [[UV Stabilizers in plastics]] |
|||
* [[Wood's lamp]] |
|||
== References == |
|||
{{reflist|colwidth=30em}} |
|||
== Further reading == |
|||
{{commons cat|Ultraviolet light}} |
|||
{{Wiktionary}} |
|||
* {{Citation |
|||
| last = Hu |
|||
| first = S |
|||
| last2 = Ma |
|||
| first2 = F |
|||
| last3 = Collado-Mesa |
|||
| first3 = F |
|||
| last4 = Kirsner |
|||
| first4 = R. S. |
|||
| title = UV radiation, latitude, and melanoma in US Hispanics and blacks |
|||
| journal = Arch. Dermatol. |
|||
| volume = 140 |
|||
| issue = 7 |
|||
| pages = 819–824 |
|||
| month = Jul |
|||
| year = 2004 |
|||
| url = http://archderm.ama-assn.org/cgi/content/full/140/7/819 |
|||
| doi = 10.1001/archderm.140.7.819 |
|||
| id = |
|||
| pmid = 15262692 }} |
|||
* {{Citation |
|||
| last = Hockberger |
|||
| first = Philip E. |
|||
| title = A History of Ultraviolet Photobiology for Humans, Animals and Microorganisms |
|||
| journal =Photochemisty and Photobiology |
|||
| volume =76 |
|||
| issue =6 |
|||
| pages =561–569 |
|||
| date = |
|||
| year =2002 |
|||
| url =http://www.bioone.org/archive/0031-8655/76/6/pdf/i0031-8655-76-6-561.pdf |
|||
| doi = 10.1562/0031-8655(2002)076<0561:AHOUPF>2.0.CO;2|label=10.1562/0031-8655(2002)076<0561:AHOUPF>2.0.CO;2 |
|||
| format = {{Dead link|date=February 2009}} – <sup>[http://scholar.google.co.uk/scholar?hl=en&lr=&q=author%3AHockberger+intitle%3AA+History+of+Ultraviolet+Photobiology+for+Humans%2C+Animals+and+Microorganisms&as_publication=Photochemisty+and+Photobiology&as_ylo=2002&as_yhi=2002&btnG=Search Scholar search]</sup> }} |
|||
* {{Citation |
|||
| last = Allen |
|||
| first = Jeannie |
|||
| title = Ultraviolet Radiation: How it Affects Life on Earth |
|||
| url = http://earthobservatory.nasa.gov/Library/UVB/ |
|||
|date=2001-09-06 |
|||
| series = Earth Observatory |
|||
| publisher = NASA, USA |
|||
}} |
|||
{{EMSpectrum}} |
|||
[[Category:Electromagnetic spectrum]] |
|||
[[Category:Printing]] |
|||
[[Category:Ultraviolet]] |
|||
[[ar:أشعة فوق بنفسجية]] |
|||
[[zh-min-nan:Chí-goā-soàⁿ]] |
|||
[[bs:Ultraljubičasto zračenje]] |
|||
[[br:Uslimestra]] |
|||
[[bg:Ултравиолетови лъчи]] |
|||
[[ca:Ultraviolat]] |
|||
[[cs:Ultrafialové záření]] |
|||
[[cy:Uwchfioled]] |
|||
[[da:Ultraviolet lys]] |
|||
[[de:Ultraviolettstrahlung]] |
|||
[[et:Ultraviolettkiirgus]] |
|||
[[es:Radiación ultravioleta]] |
|||
[[eo:Ultraviola radiado]] |
|||
[[eu:Ultramore]] |
|||
[[fa:فرابنفش]] |
|||
[[fr:Ultraviolet]] |
|||
[[gl:Ultravioleta]] |
|||
[[ko:자외선]] |
[[ko:자외선]] |
||
[[hi:पराबैंगनी]] |
[[hi:पराबैंगनी]] |
||
Revision as of 10:07, 10 June 2009

Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than x-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV. It is so named because the spectrum consists of electromagnetic waves with frequencies higher than those that humans identify as the color violet.
UV light is found in sunlight and is emitted by electric arcs and specialized lights such as black lights. As an ionizing radiation it can cause chemical reactions, and causes many substances to glow or fluoresce. Most people are aware of the effects of UV through the painful condition of sunburn, but the UV spectrum has many other effects, both beneficial and damaging, on human health.
Discovery
The discovery of UV radiation was intimately associated with the observation that silver salts darken when exposed to sunlight. In 1801 the German physicist Johann Wilhelm Ritter made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum were especially effective at darkening silver chloride-soaked paper. He called them "de-oxidizing rays" to emphasize their chemical reactivity and to distinguish them from "heat rays" at the other end of the visible spectrum. The simpler term "chemical rays" was adopted shortly thereafter, and it remained popular throughout the 19th century. The terms chemical and heat rays were eventually dropped in favor of ultraviolet and infrared radiation, respectively.[1]
The discovery of the ultraviolet radiation below 200 nm, named vacuum ultraviolet because it is strongly absorbed by air, was made in 1893 by the German physicist Victor Schumann.[2]
Origin of term
The name means "beyond violet" (from Latin ultra, "beyond"), violet being the color of the shortest wavelengths of visible light. UV light has a shorter wavelength than that of violet light.
Subtypes
The electromagnetic spectrum of ultraviolet light can be subdivided in a number of ways. The draft ISO standard on determining solar irradiances (ISO-DIS-21348)[3] describes the following ranges:
| Name | Abbreviation | Wavelength range in nanometers | Energy per photon |
|---|---|---|---|
| Ultraviolet A, long wave, or black light | UVA | 400 nm–320 nm | 3.10–3.94 eV |
| Near | NUV | 400 nm–300 nm | 3.10–4.13 eV |
| Ultraviolet B or medium wave | UVB | 320 nm–280 nm | 3.94–4.43 eV |
| Middle | MUV | 300 nm–200 nm | 4.13–6.20 eV |
| Ultraviolet C, short wave, or germicidal | UVC | 280 nm–100 nm | 4.43–12.4 eV |
| Far | FUV | 200 nm–122 nm | 6.20–10.2 eV |
| Vacuum | VUV | 200 nm–10 nm | 6.20–124 eV |
| Extreme | EUV | 121 nm–10 nm | 10.2–124 eV |
In photolithography and laser technology, the term deep ultraviolet or DUV refers to wavelengths below 300 nm. "Vacuum UV" is so named because it is absorbed strongly by air and is therefore used in a vacuum. In the long-wave limit of this region, roughly 150–200 nm, the principal absorber is the oxygen in air. Work in this region can be performed in an oxygen free atmosphere, pure nitrogen being commonly used, which avoids the need for a vacuum chamber.
See 1 E-7 m for a list of objects of comparable sizes.
Black light
A black light, or Wood's light, is a lamp that emits long wave UV radiation and very little visible light. Commonly these are referred to as simply a "UV light". Fluorescent black lights are typically made in the same fashion as normal fluorescent lights except that only one phosphor is used and the normally clear glass envelope of the bulb may be replaced by a deep-bluish-purple glass called Wood's glass, a nickel-oxide–doped glass, which blocks almost all visible light above 400 nanometers. The color of such lamps is often referred to in the trade as "blacklight blue" or "BLB." This is to distinguish these lamps from "bug zapper" blacklight ("BL") lamps that don't have the blue Wood's glass. The phosphor typically used for a near 368 to 371 nanometer emission peak is either europium-doped strontium fluoroborate (SrB4O7F:Eu2+) or europium-doped strontium borate (SrB4O7:Eu2+) while the phosphor used to produce a peak around 350 to 353 nanometers is lead-doped barium silicate (BaSi2O5:Pb+). "Blacklight Blue" lamps peak at 365 nm.
While "black lights" do produce light in the UV range, their spectrum is confined to the longwave UVA region. Unlike UVB and UVC, which are responsible for the direct DNA damage that leads to skin cancer, black light is limited to lower energy, longer waves and does not cause sunburn. However, UVA is capable of causing damage to collagen fibers and destroying vitamin A in skin.
A black light may also be formed by simply using Wood's glass instead of clear glass as the envelope for a common incandescent bulb. This was the method used to create the very first black light sources. Though it remains a cheaper alternative to the fluorescent method, it is exceptionally inefficient at producing UV light (less than 0.1% of the input power) owing to the black body nature of the incandescent light source. Incandescent UV bulbs, due to their inefficiency, may also become dangerously hot during use. More rarely still, high power (hundreds of watts) mercury vapor black lights can be found which use a UV emitting phosphor and an envelope of Wood's glass. These lamps are used mainly for theatrical and concert displays and also become very hot during normal use.
Some UV fluorescent bulbs specifically designed to attract insects for use in bug zappers use the same near-UV emitting phosphor as normal blacklights, but use plain glass instead of the more expensive Wood's glass. Plain glass blocks less of the visible mercury emission spectrum, making them appear light blue to the naked eye. These lamps are referred to as "blacklight" or "BL" in most lighting catalogs.
Ultraviolet light can also be generated by some light-emitting diodes.
Natural sources of UV
The Sun emits ultraviolet radiation in the UVA, UVB, and UVC bands, but because of absorption in the atmosphere's ozone layer, 98.7% of the ultraviolet radiation that reaches the Earth's surface is UVA. (Some of the UVB and UVC radiation is responsible for the generation of the ozone layer.)
Ordinary glass is partially transparent to UVA but is opaque to shorter wavelengths while Silica or quartz glass, depending on quality, can be transparent even to vacuum UV wavelengths. Ordinary window glass passes about 90% of the light above 350 nm, but blocks over 90% of the light below 300 nm.[4][5][6]
The onset of vacuum UV, 200 nm, is defined by the fact that ordinary air is opaque at shorter wavelengths. This opacity is due to the strong absorption of light of these wavelengths by oxygen in the air. Pure nitrogen (less than about 10 ppm oxygen) is transparent to wavelengths in the range of about 150–200 nm. This has wide practical significance now that semiconductor manufacturing processes are using wavelengths shorter than 200 nm. By working in oxygen-free gas, the equipment does not have to be built to withstand the pressure differences required to work in a vacuum. Some other scientific instruments, such as circular dichroism spectrometers, are also commonly nitrogen purged and operate in this spectral region.
Extreme UV is characterized by a transition in the physics of interaction with matter: wavelengths longer than about 30 nm interact mainly with the chemical valence electrons of matter, while wavelengths shorter than that interact mainly with inner shell electrons and nuclei. The long end of the EUV/XUV spectrum is set by a prominent He+ spectral line at 30.4 nm. XUV is strongly absorbed by most known materials, but it is possible to synthesize multilayer optics that reflect up to about 50% of XUV radiation at normal incidence. This technology has been used to make telescopes for solar imaging; it was pioneered by the NIXT and MSSTA sounding rockets in the 1990s; (current examples are SOHO/EIT and TRACE) and for nanolithography (printing of traces and devices on microchips).
Human health-related effects of UV radiation
Beneficial effects
Vitamin D
The Earth's atmosphere blocks UV radiation from penetrating through the atmosphere by 98.7%. A positive effect of UVB exposure is that it induces the production of vitamin D in the skin. It has been estimated that tens of thousands of premature deaths occur in the United States annually from a range of cancers due to vitamin D deficiency.[7] Another effect of vitamin D deficiency is poor absorption of calcium which can lead to bone diseases.
Some studies show most people get adequate Vitamin D through food and incidental exposure.[8]
Many countries have fortified certain foods with Vitamin D to prevent deficiency. Eating fortified foods or taking a dietary supplement pill is usually preferred to UVB exposure, due to the increased risk of skin cancer from UV radiation.[8]
Aesthetics
Too little UVB radiation leads to a lack of Vitamin D. Too much UVB radiation leads to direct DNA damages and sunburn. An appropriate amount of UVB (which varies according to skin color) leads to a limited amount of direct DNA damage. This is recognized and repaired by the body. Then the melanin production is increased which leads to a long lasting tan. This tan occurs with a 2 day lag phase after irradiation, but it is much less harmful and long lasting than the one obtained from UVA. However some tanning lotions and sprays available in the market don't require UV exposure.
Medical applications
Ultraviolet radiation has other medical applications, in the treatment of skin conditions such as psoriasis and vitiligo. UVA radiation can be used in conjunction with psoralens (PUVA treatment). UVB radiation is rarely used in conjunction with psoralens. In cases of psoriasis and vitiligo, UV light with wavelength of 311 nm is most effective.[citation needed]
Harmful effects
An overexposure to UVB radiation can cause sunburn and some forms of skin cancer. In humans, prolonged exposure to solar UV radiation may result in acute and chronic health effects on the skin, eye, and immune system.[9] However the most deadly form - malignant melanoma - is mostly caused by the indirect DNA damage (free radicals and oxidative stress). This can be seen from the absence of a UV-signature mutation in 92% of all melanoma.[10]
UVC rays are the highest energy, most dangerous type of ultraviolet light. Little attention has been given to UVC rays in the past since they are filtered out by the atmosphere. However, their use in equipment such as pond sterilization units may pose an exposure risk, if the lamp is switched on outside of its enclosed pond sterilization unit.

Skin
Ultraviolet (UV) irradiation present in sunlight is an environmental human carcinogen. The toxic effects of UV from natural sunlight and therapeutic artificial lamps are a major concern for human health. The major acute effects of UV irradiation on normal human skin comprise sunburn inflammation erythema, tanning, and local or systemic immunosuppression.
— Matsumura and Ananthaswamy, (2004)[11]
UVA, UVB and UVC can all damage collagen fibers and thereby accelerate aging of the skin. Both UVA and UVB destroy vitamin A in skin which may cause further damage.[12] In the past, UVA was considered less harmful, but today it is known that it can contribute to skin cancer via indirect DNA damage (free radicals and reactive oxygen species). It penetrates deeply but it does not cause sunburn. UVA does not damage DNA directly like UVB and UVC, but it can generate highly reactive chemical intermediates, such as hydroxyl and oxygen radicals, which in turn can damage DNA. Because it does not cause reddening of the skin (erythema) it cannot be measured in SPF testing. There is no good clinical measurement for blockage of UVA radiation, but it is important that sunscreen block both UVA and UVB. Some scientists blame the absence of UVA filters in sunscreens for the higher melanoma-risk that was found for sunscreen users.[13]

UVB light can cause direct DNA damage. The radiation excites DNA molecules in skin cells, causing aberrant covalent bonds to form between adjacent cytosine bases, producing a dimer. When DNA polymerase comes along to replicate this strand of DNA, it reads the dimer as "AA" and not the original "CC". This causes the DNA replication mechanism to add a "TT" on the growing strand. This is a mutation, which can result in cancerous growths and is known as a "classical C-T mutation". The mutations that are caused by the direct DNA damage carry a UV signature mutation that is commonly seen in skin cancers. The mutagenicity of UV radiation can be easily observed in bacteria cultures. This cancer connection is one reason for concern about ozone depletion and the ozone hole. UVB causes some damage to collagen but at a very much slower rate than UVA.
As a defense against UV radiation, the amount of the brown pigment melanin in the skin increases when exposed to moderate (depending on skin type) levels of radiation; this is commonly known as a sun tan. The purpose of melanin is to absorb UV radiation and dissipate the energy as harmless heat, blocking the UV from damaging skin tissue. UVA gives a quick tan that lasts for days by oxidizing melanin that was already present and triggers the release of the melanin from melanocytes. UVB yields a tan that takes roughly 2 days to develop because it stimulates the body to produce more melanin. The photochemical properties of melanin make it an excellent photoprotectant. However, sunscreen chemicals can not dissipate the energy of the excited state as efficiently as melanin and therefore the penetration of sunscreen ingredients into the lower layers of the skin is increasing the amount of free radicals and reactive oxygen species (ROS).[14]
Sunscreen prevents the direct DNA damage which causes sunburn. Most of these products contain an SPF rating to show how well they block UVB rays. The SPF rating, however, offers no data about UVA protection. In the US, the Food and Drug Administration is considering adding a star rating system to show UVA protection. A similar system is already used in some European countries.[citation needed]
Some sunscreen lotions now include compounds such as titanium dioxide which helps protect against UVA rays. Other UVA blocking compounds found in sunscreen include zinc oxide and avobenzone. Cantaloupe extract, rich in the compound superoxide dismutase (SOD), can be bound with gliadin to form glisodin, an orally-effective protectant against UVB radiation. There are also naturally occurring compounds found in rainforest plants that have been known to protect the skin from UV radiation damage, such as the fern Phlebodium aureum.
Sunscreen safety debate
Medical organizations recommend that patients protect themselves from UV radiation using sunscreen. Five sunscreen ingredients have been shown to protect mice against skin tumors (see sunscreen).
However, some sunscreen chemicals produce potentially harmful substances if they are illuminated while in contact with living cells.[15][16][17] The amount of sunscreen which penetrates through the stratum corneum may or may not be large enough to cause damage. In one study of sunscreens, the authors write:[18]
The question whether UV filters acts on or in the skin has so far not been fully answered. Despite the fact that an answer would be a key to improve formulations of sun protection products, many publications carefully avoid addressing this question.
In an experiment by Hanson et al. that was published in 2006, the amount of harmful reactive oxygen species (ROS) was measured in untreated and in sunscreen treated skin. In the first 20 minutes the film of sunscreen had a protective effect and the number of ROS species was smaller. After 60 minutes, however, the amount of absorbed sunscreen was so high, that the amount of ROS was higher in the sunscreen treated skin than in the untreated skin.[14]
Eye
High intensities of UVB light are hazardous to the eyes, and exposure can cause welder's flash (photokeratitis or arc eye) and may lead to cataracts, pterygium,[19][20] and pinguecula formation.
UV light is absorbed by molecules known as chromophores, which are present in the eye cells and tissues. Chromophores absorb light energy from the various wavelengths at different rates - a pattern known as absorption spectrum. If too much UV light is absorbed, eye structures such as the cornea, the lens and the retina can be damaged.[21]
Protective eyewear is beneficial to those who are working with or those who might be exposed to ultraviolet radiation, particularly short wave UV. Given that light may reach the eye from the sides, full coverage eye protection is usually warranted if there is an increased risk of exposure, as in high altitude mountaineering. Mountaineers are exposed to higher than ordinary levels of UV radiation, both because there is less atmospheric filtering and because of reflection from snow and ice.
Ordinary, untreated eyeglasses give some protection. Most plastic lenses give more protection than glass lenses, because, as noted above, glass is transparent to UVA and the common acrylic plastic used for lenses is less so. Some plastic lens materials, such as polycarbonate, inherently block most UV. There are protective treatments available for eyeglass lenses that need it which will give better protection. But even a treatment that completely blocks UV will not protect the eye from light that arrives around the lens.
Degradation of polymers, pigments and dyes

Many polymers used in consumer products are degraded by UV light, and need addition of UV absorbers to inhibit attack, especially if the products are used outdoors and so exposed to sunlight. The problem appears as discoloration or fading, cracking and sometimes, total product disintegration if cracking has proceeded far enough. The rate of attack increases with exposure time and sunlight intensity.
It is known as UV degradation, and is one form of polymer degradation. Sensitive polymers include thermoplastics, such as polypropylene and polyethylene as well as speciality fibres like aramids. UV absorption leads to chain degradation and loss of strength at sensitive points in the chain structure. They include tertiary carbon atoms, which in polypropylene occur in every repeat unit. Aramid rope must be shielded by covering it with a sheath of thermoplastic if it is to retain its strength.

In addition, many pigments and dyes absorb UV and change colour, so paintings and textiles may need extra protection both from sunlight and fluorescent bulbs, two common sources of UV radiation. Old and antique paintings such as watercolour paintings for example, usually need to be placed away from direct sunlight. Common window glass provides some protection by absorbing some of the harmful UV, but valuable artifacts need extra shielding. Many museums place black curtains over watercolour paintings and ancient textiles, for example. Since watercolours can have very low pigment levels, they need extra protection from the effects of UV light.
Blockers and absorbers
Ultraviolet Light Absorbers (UVAs) are molecules used in organic materials (polymers, paints, etc.) to absorb UV light in order to reduce the UV degradation (photo-oxidation) of a material. A number of different UVAs exist with different absorption properties. UVAs can disappear over time, so monitoring of UVA levels in weathered materials is necessary.
In sunscreen, ingredients which absorb UVA/UVB rays, such as avobenzone and octyl methoxycinnamate, are known as absorbers. They are contrasted with physical "blockers" of UV radiation such as titanium dioxide and zinc oxide. (See sunscreen for a more complete list.)
Applications of UV
Security

To help thwart counterfeiters, sensitive documents (e.g. credit cards, driver's licenses, passports) may also include a UV watermark that can only be seen when viewed under a UV-emitting light. Passports issued by most countries usually contain UV sensitive inks and security threads. Visa stamps and stickers on passports of visitors contain large and detailed seals invisible to the naked eye under normal lights, but strongly visible under UV illumination. Passports issued by many nations have UV sensitive watermarks on all pages of the passport. Currencies of various countries' banknotes have an image, as well as many multicolored fibers, that are visible only under ultraviolet light.
Some brands of pepper spray will leave an invisible chemical (UV Dye) that is not easily washed off on a pepper sprayed attacker, which would help police identify them later.[22]
Forensics
UV is an investigative tool at the crime scene helpful in locating and identifying bodily fluids (semen, blood, bile etc.)
Fluorescent lamps
Fluorescent lamps produce UV radiation by ionising low-pressure mercury vapour. A phosphorescent coating on the inside of the tubes absorbs the UV and converts it to visible light.
The main mercury emission wavelength is in the UVC range. Unshielded exposure of the skin or eyes to mercury arc lamps that do not have a conversion phosphor is quite dangerous.
The light from a mercury lamp is predominantly at discrete wavelengths. Other practical UV sources with more continuous emission spectra include xenon arc lamps (commonly used as sunlight simulators), deuterium arc lamps, mercury-xenon arc lamps, metal-halide arc lamps, and tungsten-halogen incandescent lamps.
Astronomy
In astronomy, very hot objects preferentially emit UV radiation (see Wien's law). Because the ozone layer blocks many UV frequencies from reaching telescopes on the surface of the Earth, most UV observations are made from space. (See UV astronomy, space observatory.)
Hunting
Hunters can use UV lights to follow the blood trail of a wounded deer, elk or other game animal.
Biological surveys and pest control
Some animals, including birds, reptiles, and insects such as bees, can see into the near ultraviolet. Many fruits, flowers, and seeds stand out more strongly from the background in ultraviolet wavelengths as compared to human color vision. Scorpions glow or take on a yellow to green color under UV illumination thus assisting in the control of these sometimes fatally venomous arachnids. Many birds have patterns in their plumage that are invisible at usual wavelengths but observable in ultraviolet, and the urine and other secretions of some animals, including dogs, cats, and human beings, is much easier to spot with ultraviolet. Urine trails of rodents can be detected by pest control technicians for proper treatment of infested dwellings.
Many insects use the ultraviolet wavelength emissions from celestial objects as references for flight navigation. A local ultraviolet emissor will normally disrupt the navigation process and will eventually attract the flying insect.

Ultraviolet traps called bug zappers are used to eliminate various small flying insects. They are attracted to the UV light, and are killed using an electric shock, or trapped once they come into contact with the device. Different designs of ultraviolet light traps are also used by entomologists for collecting nocturnal insects during faunistic survey studies.
Spectrophotometry
UV/VIS spectroscopy is widely used as a technique in chemistry, to analyze chemical structure, most notably conjugated systems. UV radiation is often used in visible spectrophotometry to determine the existence of fluorescence in a given sample.
Sanitary compliance
UV lamps including newer LEDs (light emitting diode) aid in the detection of organic mineral deposits that remain on surfaces where periodic cleaning and sanitizing may not be properly accomplished. Both urine and phosphate soaps are easily detected using UV inspection. Pet urine deposits in carpeting or other hard surfaces can be detected for accurate treatment and removal of mineral tracers and the odor causing bacteria that feeds on proteins within. Many hotel and hospitality industries use UV lamp to inspect for unsanitary bedding to determine life cycle for mattress restoration as well as general performance of the cleaning staff.
Air purification
Using a catalytic reaction from titanium dioxide and UV light exposure, a strong oxidative effect occurs on any organic that passes through the media converting otherwise irritating pathogens, pollens, and mold spores into harmless inert byproducts.
Analyzing minerals

Ultraviolet lamps are also used in analyzing minerals, gems, and in other detective work including authentication of various collectibles. Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light; or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet.
Authentication
In other detective work including authentication of various collectibles art, and . Detecting counterfeit currency absent of marker dyes. Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light; or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet.
Chemical markers
UV fluorescent dyes are used in many applications (for example, biochemistry and forensics). The Green Fluorescent Protein (GFP) is often used in genetics as a marker. Many substances, such as proteins, have significant light absorption bands in the ultraviolet that are of use and interest in biochemistry and related fields. UV-capable spectrophotometers are common in such laboratories.
Photochemotherapy
Exposure to UVA light while the skin is hyper-photosensitive by taking psoralens is an effective treatment for psoriasis called PUVA. Due to psoralens potentially causing damage to the liver, PUVA may only be used a limited number of times over a patient's lifetime
Phototherapy
This article needs additional citations for verification. (April 2007) |
This article needs attention from an expert in health. Please add a reason or a talk parameter to this template to explain the issue with the article. (March 2008) |
Exposure to UVB light, particularly the 310 nm narrowband UVB range, is an effective long-term treatment for many skin conditions like psoriasis, vitiligo, eczema, and others.[23] UVB phototherapy does not require additional medications or topical preparations for the therapeutic benefit; only the light exposure is needed. However, phototherapy can be effective when used in conjunction with certain topical treatments such as anthralin, coal tar, and Vitamin A and D derivatives, or systemic treatments such as methotrexate and soriatane.[24]
Typical treatment regimes involve short exposure to UVB rays 3 to 5 times a week at a hospital or clinic, and up to 30 or more sessions may be required before results are noticeable. Almost all of the conditions that respond to UVB light are chronic problems, so continual treatment is required to keep those problems in check. Home UVB systems are common solutions for those whose conditions respond to treatment. Home systems permit the patient to treat themselves every other day (the ideal treatment regimen for most) without the frequent, costly trips to the office/clinic and back.
Side effects may include itching and redness of the skin due to UVB exposure, and possibly sunburn, if patients do not minimize exposure to natural UV rays during treatment days. Cataracts can frequently develop if the eyes are not protected from UVB light exposure. There is no link between an increase in the patient's risk for skin cancer and the proper use of UVB phototherapy. "Proper use" is generally defined as reaching the "Sub-Erythemic Dose" (S.E.D.), the maximum amount of UVB your skin can receive without burning.
Certain fungal growths under the toenail can be treated using a specific wavelength of UV delivered from a high power LED (light emitting diode) and can be safer than traditional systemic drugs.
Photolithography
Ultraviolet radiation is used for very fine resolution photolithography, a procedure where a chemical known as a photoresist is exposed to UV radiation which has passed through a mask. The light allows chemical reactions to take place in the photoresist, and after development (a step that either removes the exposed or unexposed photoresist), a geometric pattern which is determined by the mask remains on the sample. Further steps may then be taken to "etch" away parts of the sample with no photoresist remaining.
UV radiation is used extensively in the electronics industry because photolithography is used in the manufacture of semiconductors, integrated circuit components[25] and printed circuit boards.
Checking electrical insulation
A new application of UV is to detect corona discharge (often simply called "corona") on electrical apparatus. Degradation of insulation of electrical apparatus or pollution causes corona, wherein a strong electric field ionizes the air and excites nitrogen molecules, causing the emission of ultraviolet radiation. The corona degrades the insulation level of the apparatus. Corona produces ozone and to a lesser extent nitrogen oxide which may subsequently react with water in the air to form nitrous acid and nitric acid vapour in the surrounding air.[26]
Sterilization

Ultraviolet lamps are used to sterilize workspaces and tools used in biology laboratories and medical facilities. Commercially-available low pressure mercury-vapor lamps emit about 86% of their light at 254 nanometers (nm) which coincides very well with one of the two peaks of the germicidal effectiveness curve (i.e., effectiveness for UV absorption by DNA). One of these peaks is at about 265 nm and the other is at about 185 nm. Although 185 nm is better absorbed by DNA, the quartz glass used in commercially-available lamps, as well as environmental media such as water, are more opaque to 185 nm than 254 nm (C. von Sonntag et al., 1992). UV light at these germicidal wavelengths causes adjacent thymine molecules on DNA to dimerize, if enough of these defects accumulate on a microorganism's DNA its replication is inhibited, thereby rendering it harmless (even though the organism may not be killed outright). However, since microorganisms can be shielded from ultraviolet light in small cracks and other shaded areas, these lamps are used only as a supplement to other sterilization techniques.
Disinfecting drinking water
UV radiation can be an effective viricide and bactericide. Disinfection using UV radiation is commonly used in wastewater treatment applications and is finding an increased usage in drinking water treatment. Many bottlers of spring water use UV disinfection equipment to sterilize their water. Solar water disinfection is the process of using PET bottles and sunlight to disinfect water.
New York City has approved the construction of a 2 billion gallon per day ultraviolet drinking water disinfection facility.[27] There are also several facilities under construction and several in operation that treat waste water with several stages of filters, hydrogen peroxide and UV light to bring the water up to drinking standards. One such facility exists in Orange County, California.[28][29] NASA has examined the use of this technology, using titanium dioxide as catalyst, for breaking down harmful products in spacecraft waste water.[30]
It used to be thought that UV disinfection was more effective for bacteria and viruses, which have more exposed genetic material, than for larger pathogens which have outer coatings or that form cyst states (e.g., Giardia) that shield their DNA from the UV light. However, it was recently discovered that ultraviolet radiation can be somewhat effective for treating the microorganism Cryptosporidium. The findings resulted in the use of UV radiation as a viable method to treat drinking water. Giardia in turn has been shown to be very susceptible to UV-C when the tests were based on infectivity rather than excystation.[31] It has been found that protists are able to survive high UV-C doses but are sterilized at low doses.
Solar water disinfection[32] (SODIS) has been extensively researched in Switzerland and has proven ideal to treat small quantities of water cheaply using natural sunlight. Contaminated water is poured into transparent plastic bottles and exposed to full sunlight for six hours. The sunlight treats the contaminated water through two synergetic mechanisms: UV-A irradiation and increased water temperature. If the water temperatures rises above 50 °C, the disinfection process is three times faster.
Food processing
As consumer demand for fresh and "fresh-like" food products increases, the demand for nonthermal methods of food processing is likewise on the rise. In addition, public awareness regarding the dangers of food poisoning is also raising demand for improved food processing methods. Ultraviolet radiation is used in several food processes to kill unwanted microorganisms. UV light can be used to pasteurize fruit juices by flowing the juice over a high intensity ultraviolet light source.[33] The effectiveness of such a process depends on the UV absorbance of the juice (see Beer's law).
Fire detection
Ultraviolet detectors generally use either a solid-state device, such as one based on silicon carbide or aluminium nitride, or a gas-filled tube as the sensing element. UV detectors which are sensitive to UV light in any part of the spectrum respond to irradiation by sunlight and artificial light. A burning hydrogen flame, for instance, radiates strongly in the 185 to 260 nanometer range and only very weakly in the IR region, while a coal fire emits very weakly in the UV band yet very strongly at IR wavelengths; thus a fire detector which operates using both UV and IR detectors is more reliable than one with a UV detector alone. Virtually all fires emit some radiation in the UVC band, while the Sun's radiation at this band is absorbed by the Earth's atmosphere. The result is that the UV detector is "solar blind", meaning it will not cause an alarm in response to radiation from the Sun, so it can easily be used both indoors and outdoors.
UV detectors are sensitive to most fires, including hydrocarbons, metals, sulfur, hydrogen, hydrazine, and ammonia. Arc welding, electrical arcs, lightning, X-rays used in nondestructive metal testing equipment (though this is highly unlikely), and radioactive materials can produce levels that will activate a UV detection system. The presence of UV-absorbing gases and vapors will attenuate the UV radiation from a fire, adversely affecting the ability of the detector to detect flames. Likewise, the presence of an oil mist in the air or an oil film on the detector window will have the same effect.
Herpetology
Reptiles need long wave UV light to metabolize calcium for bone and egg production. Thus, in a typical reptile enclosure, a fluorescent UV lamp is used at one end of the enclosure for calcium absorption and a common incandescent bulb is used at the other end for heat (basking).
Curing of electronic potting resins
Electronic components that require clear transparency for light to exit or enter (photo voltaic panels and sensors)can be potted using acrylic resins that are cured using UV light energy. The advantages are low VOC emissions and rapid curing.
Curing of inks, adhesives, varnishes and coatings
Certain inks, coatings and adhesives are formulated with photoinitiators and resins. When exposed to the correct energy and irradiance in the required band of UV light, polymerization occurs, and so the adhesives harden or cure. Usually, this reaction is very quick, a matter of a few seconds. Applications include glass and plastic bonding, optical fiber coatings, the coating of flooring, UV Coating and paper finishes in offset printing, and dental fillings. Curing of decorative finger nail "gels". Industrial UV curing systems can be found at DDU Enterprises, Inc.'s website "Doctoruv.com"
An industry has developed around the manufacture of UV sources for UV curing applications. This includes UV lamps, UV LEDs and Excimer Flash lamps. Fast processes such as flexo or offset printing require high intensity light focused via reflectors onto a moving substrate and medium and high pressure Hg (mercury) or Fe (iron, doped) based bulbs are used which can be energised with electric arc or microwaves. Currently, LED-based sources (focused and not focused) are achieving comparable power levels and are expected to become as important as Hg lamps in these applications.[citation needed] Lower power sources (fluorescent lamps, LED) can be used for static applications and in some cases, small high pressure lamps can have light focused and transmitted to the work area via liquid filled or fibre optic light guides.
Deterring substance abuse in public places
This section needs additional citations for verification. (April 2007) |
UV lights have been installed in some parts of the world in public restrooms, and on public transport, for the purpose of deterring substance abuse. The blue color of these lights, combined with the fluorescence of the skin, make it harder for intravenous drug users to find a vein.[34] The efficacy of these lights for that purpose has been questioned, with some suggesting that drug users simply find a vein outside the public restroom and mark the spot with a marker for accessibility when inside the restroom. There is currently no published evidence supporting the idea of a deterrent effect.
Sun tanning
Sun tanning describes a darkening of the skin in a natural physiological response stimulated by exposure to ultraviolet radiation from sunshine (or a sunbed). With excess exposure to the sun, a suntanned area can also develop sunburn. The increased production of melanin is triggered by the direct DNA damage.[35] This kind of damage is recognized by the body and as a defense against UV radiation the skin produces more melanin. Melanin dissipates the UV energy as harmless heat, and therefore it is an excellent photoprotectant. Melanin protects against the direct DNA damage and against the indirect DNA damage. Sunscreen protects only against the direct DNA damage, but increases the indirect DNA damage[15][16][17] - this causes the higher amount of melanoma that had been found repeatedly in sunscreen users compared to non-users.[36][37][13][38][39]
Erasing EPROM modules
Some EPROM (erasable programmable read-only memory) modules are erased by exposure to UV radiation. These modules often have a transparent glass (quartz) window on the top of the chip that allows the UV radiation in. These have been largely superseded by EEPROM and flash memory chips in most devices.
Preparing low surface energy polymers
UV radiation is useful in preparing low surface energy polymers for adhesives. Polymers exposed to UV light will oxidize thus raising the surface energy of the polymer. Once the surface energy of the polymer has been raised, the bond between the adhesive and the polymer will not be smaller.
Reading otherwise illegible papyruses
Using multi-spectral imaging it is possible to read illegible papyruses, such as the burned papyruses of the Villa of the Papyri or of Oxyrhynchus, or the Archimedes palimpsest. The technique involves taking pictures of the illegible papyruses using different filters in the infrared or ultraviolet range, finely tuned to capture certain wavelengths of light. Thus, the optimum spectral portion can be found for distinguishing ink from paper on the papyrus surface.
Lasers
Ultraviolet lasers have applications in industry (laser engraving), medicine (dermatology and keratectomy), free air secure communications and computing (optical storage). They can be made by applying frequency conversion to lower-frequency lasers, or from Ce:LiSAF crystals (cerium doped with lithium strontium aluminum fluoride), a process developed in the 1990s at Lawrence Livermore National Laboratory.[40]
UV solar cells and UV degration of solar cells
Japan's National Institute of Advanced Industrial Science and Technology (AIST) has succeeded in developing a transparent solar cell that uses ultraviolet light to generate electricity but allows visible light to pass through it. Most conventional solar cells use visible and infrared light to generate electricity. In contrast, the innovative new solar cell uses ultraviolet radiation. Used to replace conventional window glass, the installation surface area could be large, leading to potential uses that take advantage of the combined functions of power generation, lighting and temperature control.[41]
Also PEDOT-PSS solar cells is a ultraviolet (UV) light selective and sensitive photovoltaic cell easily fabricated.[42]
On the other hand, a nanocrystalline layer of Cu2O in the construction of photovoltaic cells increases their ability to utilize UV radiations for photocurrent generation.[43]
Non-destructive Testing
UV light of a specified spectrum and intensity is used to stimulate fluorescent dyes so as to highlight defects in a broad range of materials. These dyes may be carried into surface-breaking defects by capillary action (liquid penetrant inspection) or they may be bound to ferrite particles caught in magnetic leakage fields in ferrous materials (magnetic particle inspection).
This section needs expansion. You can help by adding to it. (November 2008) |
Evolutionary significance
Evolution of early reproductive proteins and enzymes is attributed in modern models of evolutionary theory to ultraviolet light. UVB light causes thymine base pairs next to each other in genetic sequences to bond together into thymine dimers, a disruption in the strand which reproductive enzymes cannot copy (see picture above). This leads to frameshifting during genetic replication and protein synthesis, usually killing the organism. As early prokaryotes began to approach the surface of the ancient oceans, before the protective ozone layer had formed, blocking out most wavelengths of UV light, they almost invariably died out. The few that survived had developed enzymes which verified the genetic material and broke up thymine dimer bonds, known as excision repair enzymes. Many enzymes and proteins involved in modern mitosis and meiosis are similar to excision repair enzymes, and are believed to be evolved modifications of the enzymes originally used to overcome UV light.[44]
See also
- Black light
- High-energy visible light
- Infrared light
- Polymer degradation
- Sun tanning
- Tanning lamp
- Titanium dioxide
- Ultraviolet photography
- UV degradation
- UV index
- UV Stabilizers in plastics
- Wood's lamp
References
- ^ Hockberger, P. E. (2002), "A history of ultraviolet photobiology for humans, animals and microorganisms", Photochem. Photobiol., 76: 561–579, doi:10.1562/0031-8655(2002)076<0561:AHOUPF>2.0.CO;2
- ^ The ozone layer protects humans from this. Lyman, T. (1914), "Victor Schumann", Astrophysical Journal, 38: 1–4, doi:10.1086/142050
- ^ "ISO 21348 Process for Determining Solar Irradiances".
- ^ "Soda Lime Glass Transmission Curve".
- ^ "B270-Superwite Glass Transmission Curve".
- ^ "Selected Float Glass Transmission Curve".
- ^ Grant, W. B. (2002). "An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation". Cancer Volume 94, Issue 6, pp. 1867-1875.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b The Science of Sun Protection, Talk of the Nation Science Friday, 24 June 2005. Vitamin D pills recommended over sun exposure, but most people in Australia and Canada get enough Vitamin D by incidental exposure, studies show.
- ^ "Health effects of UV radiation".
- ^ Davies H.; Bignell G. R.; Cox C.; (2002). "Mutations of the BRAF gene in human cancer". Nature. 417: 949–954. doi:10.1038/nature00766.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Matsumu, Y.; Ananthaswamy, H. N. (2004), "Toxic effects of ultraviolet radiation on the skin", Toxicology and Applied Pharmacology, 195 (3): 298–308, doi:10.1016/j.taap.2003.08.019
- ^ Torma, H; Berne, B; Vahlquist, A (1988), "UV irradiation and topical vitamin A modulate retinol esterification in hairless mouse epidermis", Acta Derm. Venereol., 68 (4): 291--299
- ^ a b Autier P; Dore J F; Schifflers E; et al. (1995). "Melanoma and use of sunscreens: An EORTC case control study in Germany, Belgium and France". Int. J. Cancer. 61: 749–755. doi:10.1002/ijc.2910610602.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "Autier" was defined multiple times with different content (see the help page). - ^ a b Hanson Kerry M.; Gratton Enrico; Bardeen Christopher J. (2006). "Sunscreen enhancement of UV-induced reactive oxygen species in the skin". Free Radical Biology and Medicine. 41 (8): 1205–1212. doi:10.1016/j.freeradbiomed.2006.06.011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Xu, C.; Green, Adele; Parisi, Alfio; Parsons, Peter G (2001). "Photosensitization of the Sunscreen Octyl p-Dimethylaminobenzoate b UVA in Human Melanocytes but not in Keratinocytes". Photochemistry and Photobiology. 73 (6): 600–604. doi:10.1562/0031-8655(2001)073<0600:POTSOP>2.0.CO;2.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ a b Knowland, John; McKenzie, Edward A.; McHugh, Peter J.; Cridland, Nigel A. (1993). "Sunlight-induced mutagenicity of a common sunscreen ingredient". FEBS Letters. 324(3): 309–313. doi:10.1016/0014-5793(93)80141-G.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Damiani, E.; Greci, L.; Parsons, R.; Knowland (1999). "Nitroxide radicals protect DNA from damage when illuminated in vitro in the presence of dibenzoylmethane and a common sunscreen ingredient". Free Radic. Biol. Med. 26: 809–816. doi:10.1016/S0891-5849(98)00292-5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chatelaine, E.; Gabard, B.; Surber, C. (2003) pdf Skin Penetration and Sun Protection Factor of Five UV Filters: Effect of the Vehicle, Skin Pharmacol. Appl. Skin Physiol., 16:28-35 DOI: 10.1159/000068291
- ^ Nolan, T. M.; et al. (2003). "The Role of Ultraviolet Irradiation and Heparin-Binding Epidermal Growth Factor-Like Growth Factor in the Pathogenesis of Pterygium". American Journal of Pathology.
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ^ Di Girolamo, N.; et al. (2005). "Epidermal Growth Factor Receptor Signaling Is Partially Responsible for the Increased Matrix Metalloproteinase-1 Expression in Ocular Epithelial Cells after UVB Radiation". American Journal of Pathology. 167 (2): 489–503. PMID 16049334.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - ^ "The "Burning" Facts of UV Light". SunGlassesUK.com. Retrieved 2009-04-16.
- ^ "Pepper Spray FAQ".
- ^ UV is used to treat tuberculosis of the skin
- ^ "UVB Phototherapy". National Psoriasis Foundation, USA. Archived from the original (php) on 2007-06-22. Retrieved 2007-09-23.
- ^ "Deep UV Photoresists".
- ^ "Corona - The Daytime UV Inspection Magazine".
- ^ Donna Portoti; et al. "UV Disinfection for New York City: Bridging Design with Operational Strategies" (PDF). American Water Works Association. Retrieved 2008-12-28.
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ^ Sewage in O.C. goes full circle - Los Angeles Times
- ^ New Purification Plant Answers California's Water Crisis
- ^ Antoniou, Maria G. (30 June 2007). "Application of immobilized titanium dioxide photocatalysts for the degradation of creatinine and phenol, model organic contaminants found in NASA's spacecrafts wastewater streams". Catalysis Today. 124 (3–4). Elsevier: 215–223. doi:10.1016/j.cattod.2007.03.054.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ware, M. W.; et al. "Inactivation of Giardia muris by Low Pressure Ultraviolet Light" (PDF). United States Environmental Protection Agency. Retrieved 2008-12-28.
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ^ Solar Water Disinfection
- ^ https://www.rulfsorchard.com/index.php?option=com_rsgallery2&page=inline&id=8&Itemid=34
- ^ Public toilets' lighting has wrong effect from Coventry Telegraph
- ^ Nita Agar; Antony R. Young (2005). "Review: Melanogenesis: a photoprotective response to DNA damage?". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 571 (1–2): 121–132. doi:10.1016/j.mrfmmm.2004.11.016. PMID 10.1016/j.mrfmmm.2004.11.016.
{{cite journal}}: Check|pmid=value (help)CS1 maint: multiple names: authors list (link) - ^ Garland C, Garland F, Gorham E (1992). "Could sunscreens increase melanoma risk?". Am J Public Health. 82 (4): 614–5. doi:10.2105/AJPH.82.4.614. PMID 1546792.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Westerdahl J; Ingvar C; Masback A; Olsson H (2000). "Sunscreen use and malignant melanoma". International journal of cancer. Journal international du cancer. 87: 145–50. doi:10.1002/1097-0215(20000701)87:1<145::AID-IJC22>3.0.CO;2-3.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weinstock, M. A. (1999). "Do sunscreens increase or decrease melanoma risk: An epidemiologic evaluation". Journal of Investigative Dermatology Symposium Proceedings. 4: 97–100. doi:10.1038/sj.jidsp.
{{cite journal}}: Check|doi=value (help) - ^ Vainio, H., Bianchini, F. (2000). "Cancer-preventive effects of sunscreens are uncertain". Scandinavian Journal of Work Environment and Health. 26: 529–31.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marshall, Chris (1996). "A simple, reliable ultraviolet laser: the Ce:LiSAF". Lawrence Livermore National Laboratory. Retrieved 2008-01-11.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ http://www.japanfs.org/en/pages/025337.html
- ^ http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=APPLAB000083000011002097000001&idtype=cvips&gifs=yes
- ^ Photovoltaic cell using stable Cu2O nanocrystals and conductive polymers - Patent 6849798
- ^ Margulis, Lynn and Sagan, Dorion (1986). "Origins of Sex: Three Billion Years of Genetic Recombination" (book). 1. Yale University Press.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link)
Further reading
- Hu, S; Ma, F; Collado-Mesa, F; Kirsner, R. S. (2004), "UV radiation, latitude, and melanoma in US Hispanics and blacks", Arch. Dermatol., 140 (7): 819–824, doi:10.1001/archderm.140.7.819, PMID 15262692
{{citation}}: Unknown parameter|month=ignored (help) - Hockberger, Philip E. (2002), "A History of Ultraviolet Photobiology for Humans, Animals and Microorganisms" ([dead link] – Scholar search), Photochemisty and Photobiology, 76 (6): 561–569, doi:10.1562/0031-8655(2002)076<0561:AHOUPF>2.0.CO;2
{{citation}}: External link in|format=|label=ignored (help) - Allen, Jeannie (2001-09-06), Ultraviolet Radiation: How it Affects Life on Earth, Earth Observatory, NASA, USA