Alternative complement pathway: Difference between revisions
logic |
DrComplement (talk | contribs) |
||
| Line 22: | Line 22: | ||
Since C3b is free and abundant in the plasma, it can bind to either a host cell or a pathogen surface. To prevent complement activation from proceeding on the host cell, there are several different kinds of regulatory proteins that disrupt the complement activation process: |
Since C3b is free and abundant in the plasma, it can bind to either a host cell or a pathogen surface. To prevent complement activation from proceeding on the host cell, there are several different kinds of regulatory proteins that disrupt the complement activation process: |
||
* Complement Receptor 1 (CR1 or [[CD35]]) and DAF ([[decay accelerating factor]] also known as [[CD55]]) compete with Factor B in binding with C3b on the cell surface and can even remove Bb from an already formed C3bBb complex |
* Complement Receptor 1 (CR1 or [[CD35]]) and DAF ([[decay accelerating factor]] also known as [[CD55]]) compete with Factor B in binding with C3b on the cell surface and can even remove Bb from an already formed C3bBb complex |
||
* The formation of a C3 convertase can also be prevented when a plasma protease called [[Factor I]] cleaves C3b into its inactive form, iC3b. Factor I |
* The formation of a C3 convertase can also be prevented when a plasma protease called [[Factor I]] cleaves C3b into its inactive form, iC3b. Factor I requires a C3b-binding protein cofactor such as [[Complement Factor H]], CR1 and Membrane Cofactor of Proteolysis (MCP or [[CD46]]) |
||
* [[Complement Factor H]] can inhibit the formation of the C3 convertase by competing with factor B for binding to C3b<ref>{{cite journal |author=Conrad DH, Carlo JR, Ruddy S |title=Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding |journal=J. Exp. Med. |volume=147 |issue=6 |pages=1792–1805 |year=1978 |month=June |pmid=567241 |pmc=2184316 |doi= |url=}}</ref>; accelerate the decay of the C3 convertase<ref>{{cite journal |author=Weiler JM, Daha MR, Austen KF, Fearon DT |title=Control of the amplification convertase of complement by the plasma protein beta1H |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=73 |issue=9 |pages=3268–72 |year=1976 |month=September |pmid=1067618 |pmc=431003 |doi= |url=}}</ref>; and act as a cofactor for [[Factor I]]-mediated cleavage of C3b<ref>{{cite journal |author=Pangburn MK, Schreiber RD, Müller-Eberhard HJ |title=Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution |journal=J. Exp. Med. |volume=146 |issue=1 |pages=257–70 |year=1977 |month=July |pmid=301546 |pmc=2180748 |doi= |url=}}</ref>. [[Complement Factor H]] preferentially binds to vertebrate cells (because of affinity for sialic acid residues), allowing preferential protection of host (as opposed to bacterial) cells from complement-mediated damage. |
|||
* Another complement regulatory protein is [[Factor H]], which either competes with factor B, displaces Bb from the convertase, acts as a cofactor for Factor I, or preferentially binds to C3b bound to vertebrate cells (because of affinity to sialic acid residues). |
|||
* [[CFHR5]] (Complement Factor H-Related protein 5) is able to bind to act as a cofactor for [[Factor I]], has decay accelerating activity and is able to bind preferentially to C3b at host surfaces<ref>{{cite journal |author=McRae JL, Duthy TG, Griggs KM, ''et al.'' |title=Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein |journal=J. Immunol. |volume=174 |issue=10 |pages=6250–6 |year=2005 |month=May |pmid=15879123 |doi= |url=}}</ref>. |
|||
==See also== |
==See also== |
||
Revision as of 14:32, 26 August 2010
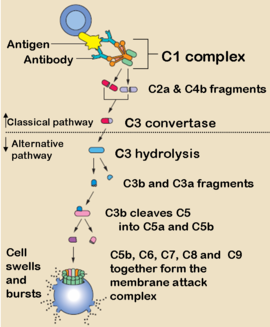

The alternative pathway of the complement system is an innate component of the immune system's natural defense against infections, which can operate without antibody participation.
The alternative pathway is one of three complement pathways that opsonize and kill pathogens. The alternative pathway does not require a specific antibody to commence, and, so, can be effective much faster than if antibody synthesis had to take place, as in the classical pathway. The caveat of this faster activation is that only specific types of antigens can activate this pathway.
Cascade
It is initiated by the spontaneous hydrolysis of C3, which is abundant in the plasma in the blood. "Tickover" occurs through the spontaneous cleavage of the thioester bond in C3 to form C3(H2O).
This change in shape allows the binding of plasma protein Factor B, which allows Factor D to cleave Factor B into Ba and Bb.
Bb remains part of the C3(H2O) to form C3(H2O)Bb. This complex is also known as a fluid-phase C3 convertase. This convertase, although only produced in small amounts, can cleave multiple C3 proteins into C3a and C3b.
The alternative pathway C3-convertase consists of the activated B and D factors, forming an unstable compound that can become stable after binding properdin, a serum protein.
After the creation of C3 convertase, the complement system follows the same path regardless of the means of activation (alternative, classical, or MBL). Binding of another C3b-fragment to the C3-convertase of the alternative pathway creates a C5-convertase analoguous to the MBL or classical pathway.
The C5-convertase of the alternative pathway consists of C3bBbC3b also referred to as C3b2Bb (instead of C4b2a3b in the other pathways)
Regulation
Since C3b is free and abundant in the plasma, it can bind to either a host cell or a pathogen surface. To prevent complement activation from proceeding on the host cell, there are several different kinds of regulatory proteins that disrupt the complement activation process:
- Complement Receptor 1 (CR1 or CD35) and DAF (decay accelerating factor also known as CD55) compete with Factor B in binding with C3b on the cell surface and can even remove Bb from an already formed C3bBb complex
- The formation of a C3 convertase can also be prevented when a plasma protease called Factor I cleaves C3b into its inactive form, iC3b. Factor I requires a C3b-binding protein cofactor such as Complement Factor H, CR1 and Membrane Cofactor of Proteolysis (MCP or CD46)
- Complement Factor H can inhibit the formation of the C3 convertase by competing with factor B for binding to C3b[1]; accelerate the decay of the C3 convertase[2]; and act as a cofactor for Factor I-mediated cleavage of C3b[3]. Complement Factor H preferentially binds to vertebrate cells (because of affinity for sialic acid residues), allowing preferential protection of host (as opposed to bacterial) cells from complement-mediated damage.
- CFHR5 (Complement Factor H-Related protein 5) is able to bind to act as a cofactor for Factor I, has decay accelerating activity and is able to bind preferentially to C3b at host surfaces[4].
See also
Further reading
- Immunobiology. Janeway, et al. 5th 3ed. ISBN 0-8153-4101-6. (5th ed. text online at [1].)
- BioCarta's diagram of the alternative pathway, [2]
External links
- ^ Conrad DH, Carlo JR, Ruddy S (1978). "Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding". J. Exp. Med. 147 (6): 1792–1805. PMC 2184316. PMID 567241.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Weiler JM, Daha MR, Austen KF, Fearon DT (1976). "Control of the amplification convertase of complement by the plasma protein beta1H". Proc. Natl. Acad. Sci. U.S.A. 73 (9): 3268–72. PMC 431003. PMID 1067618.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pangburn MK, Schreiber RD, Müller-Eberhard HJ (1977). "Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution". J. Exp. Med. 146 (1): 257–70. PMC 2180748. PMID 301546.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ McRae JL, Duthy TG, Griggs KM; et al. (2005). "Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein". J. Immunol. 174 (10): 6250–6. PMID 15879123.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
