Neutron: Difference between revisions
m Reverted edits by 106.67.85.86 (talk) to last version by Vsmith |
→Stability and beta decay: Add the other minor branches |
||
| Line 67: | Line 67: | ||
Under the [[Standard Model]] of particle physics, because the neutron consists of three [[quark]]s, the only possible decay mode without a change of [[baryon number]] is for one of the quarks to [[flavour changing processes|change]] [[flavour (physics)|flavour]] via the [[weak interaction]]. The neutron consists of two [[down quark]]s with charge −{{frac|1|3}} [[elementary charge|e]] and one [[up quark]] with charge +{{frac|2|3}} e, and the decay of one of the down quarks into a lighter up quark can be achieved by the emission of a [[W boson]]. By this means the neutron decays into a [[proton]] (which contains one down and two up quarks), an [[electron]], and an [[electron neutrino|electron antineutrino]]. |
Under the [[Standard Model]] of particle physics, because the neutron consists of three [[quark]]s, the only possible decay mode without a change of [[baryon number]] is for one of the quarks to [[flavour changing processes|change]] [[flavour (physics)|flavour]] via the [[weak interaction]]. The neutron consists of two [[down quark]]s with charge −{{frac|1|3}} [[elementary charge|e]] and one [[up quark]] with charge +{{frac|2|3}} e, and the decay of one of the down quarks into a lighter up quark can be achieved by the emission of a [[W boson]]. By this means the neutron decays into a [[proton]] (which contains one down and two up quarks), an [[electron]], and an [[electron neutrino|electron antineutrino]]. |
||
==== Free neutron decay ==== |
|||
Outside the nucleus, free neutrons are unstable and have a [[mean lifetime]] of {{val|881.5|1.5|u=s}} (about 14 minutes, 42 seconds); therefore the [[half-life]] for this process (which differs from the mean lifetime by a factor of {{nowrap|[[Natural logarithm|ln]](2) {{=}} 0.693}}) is {{val|611.0|1.0|u=s}} (about 10 minutes, 11 seconds).<ref name="RPP"/> Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as [[beta decay]]:<ref>[http://pdg.lbl.gov/2007/tables/bxxx.pdf Particle Data Group Summary Data Table on Baryons]</ref> |
Outside the nucleus, free neutrons are unstable and have a [[mean lifetime]] of {{val|881.5|1.5|u=s}} (about 14 minutes, 42 seconds); therefore the [[half-life]] for this process (which differs from the mean lifetime by a factor of {{nowrap|[[Natural logarithm|ln]](2) {{=}} 0.693}}) is {{val|611.0|1.0|u=s}} (about 10 minutes, 11 seconds).<ref name="RPP"/> Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as [[beta decay]]:<ref>[http://pdg.lbl.gov/2007/tables/bxxx.pdf Particle Data Group Summary Data Table on Baryons]</ref> |
||
:{{SubatomicParticle|Neutron0}} → {{SubatomicParticle|Proton+}} + {{SubatomicParticle|Electron}} + {{SubatomicParticle|Electron antineutrino}} |
:{{SubatomicParticle|Neutron0}} → {{SubatomicParticle|Proton+}} + {{SubatomicParticle|Electron}} + {{SubatomicParticle|Electron antineutrino}} |
||
A small fraction (about 1 in 1000) of free neutrons decay with the same products, but add an extra particle in the form of an emitted gamma ray: |
|||
| ⚫ | |||
:{{SubatomicParticle|Neutron0}} → {{SubatomicParticle|Proton+}} + {{SubatomicParticle|Electron}} + {{SubatomicParticle|Electron antineutrino}} + {{SubatomicParticle|gamma}} |
|||
This gamma ray may be thought of as a sort of "internal [[bremsstrahlung]]" which arises as the emitted beta particle interacts with the charge of the proton in an electromagnetic way. |
|||
Finally, a very small minority of neutron decays (about 4 per million) are so-called "two-body decays" in which the proton, electron and antineutrino are produced, but the electron fails to gain the 13.6 eV necessary energy to escape the proton, and therefore simply remains bound to it, as a neutral hydrogen atom. In this type of free neutron decay, essentially all of the decay energy is carried off by the antineutrino. |
|||
==== Bound neutron decay ==== |
|||
| ⚫ | |||
:{{SubatomicParticle|Proton+}} → {{SubatomicParticle|Neutron0}} + {{SubatomicParticle|Positron}} + {{SubatomicParticle|Electron neutrino}} |
:{{SubatomicParticle|Proton+}} → {{SubatomicParticle|Neutron0}} + {{SubatomicParticle|Positron}} + {{SubatomicParticle|Electron neutrino}} |
||
Revision as of 02:10, 29 July 2012
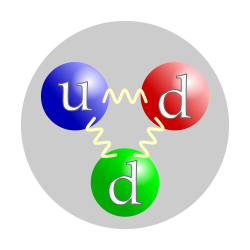 The quark structure of the neutron. (The color assignment of individual quarks is not important, only that all three colors are present.) | |
| Classification | Baryon |
|---|---|
| Composition | 1 up quark, 2 down quarks |
| Statistics | Fermionic |
| Family | Hadron |
| Interactions | Gravity, Weak, Strong, Electromagnetic |
| Symbol | n , n0 , N0 |
| Antiparticle | Antineutron |
| Theorized | Ernest Rutherford[1][2] (1920) |
| Discovered | James Chadwick[1] (1932) |
| Mass | 1.674927351(74)×10−27 kg[3] 939.565378(21) MeV/c2[3] 1.00866491600(43) u[3] |
| Mean lifetime | 881.5(15) s (free) |
| Electric charge | 0 e 0 C |
| Electric dipole moment | <2.9×10−26 e·cm |
| Electric polarizability | 1.16(15)×10−3 fm3 |
| Magnetic moment | −0.96623647(23)×10−26 J·T−1[3] −1.04187563(25)×10−3 μB[3] −1.91304272(45) μN[3] |
| Magnetic polarizability | 3.7(20)×10−4 fm3 |
| Spin | 1⁄2 |
| Isospin | 1⁄2 |
| Parity | +1 |
| Condensed | I(JP) = 1⁄2(1⁄2+) |
The neutron is a subatomic hadron particle which has the symbol
n
or
n0
, no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of protons in a nucleus is the atomic number and defines the type of element the atom forms. Neutrons are necessary within an atomic nucleus as they bind with protons via the nuclear force; protons are unable to bind with each other (see diproton) due to their mutual electromagnetic repulsion being stronger than the attraction of the nuclear force.[4] The number of neutrons is the neutron number and determines the isotope of an element. For example, the abundant carbon-12 isotope has 6 protons and 6 neutrons, while the very rare radioactive carbon-14 isotope has 6 protons and 8 neutrons.
While bound neutrons in stable nuclei are stable, free neutrons are unstable; they undergo beta decay with a mean lifetime of just under 15 minutes (881.5±1.5 s).[5] Free neutrons are produced in nuclear fission and fusion. Dedicated neutron sources like research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments. Even though it is not a chemical element, the free neutron is sometimes included in tables of nuclides.[6] It is then considered to have an atomic number of zero and a mass number of one, and is sometimes referred to as neutronium.[citation needed]
The neutron has been the key to nuclear power production. After the neutron was discovered in 1932, it was realized in 1933 that it might mediate a nuclear chain reaction. In the 1930s, neutrons were used to produce many different types of nuclear transmutations. When nuclear fission was discovered in 1938, it was soon realized that this might be the mechanism to produce the neutrons for the chain reaction, if the process also produced neutrons, and this was proven in 1939, making the path to nuclear power production evident. These events and findings led directly to the first man-made nuclear chain reaction which was self-sustaining (Chicago Pile-1, 1942) and to the first nuclear weapons (1945).
Discovery
In 1920, Ernest Rutherford conceived the possible existence of the neutron.[2] In particular, Rutherford considered that the disparity found between the atomic number of an atom and its atomic mass could be explained by the existence of a neutrally charged particle within the atomic nucleus. He considered the neutron to be a neutral double consisting of an electron orbiting a proton.[7]
In 1930 Viktor Ambartsumian and Dmitri Ivanenko in USSR found that, contrary to the prevailing opinion of the time, the nucleus cannot consist of protons and electrons. They proved that some neutral particles must be present besides the protons.[8]
In 1931, Walther Bothe and Herbert Becker in Germany found that if the very energetic alpha particles emitted from polonium fell on certain light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. At first this radiation was thought to be gamma radiation, although it was more penetrating than any gamma rays known, and the details of experimental results were very difficult to interpret on this basis.[9][10] The next important contribution was reported in 1932 by Irène Joliot-Curie and Frédéric Joliot in Paris.[11] They showed that if this unknown radiation fell on paraffin, or any other hydrogen-containing compound, it ejected protons of very high energy. This was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such a hypothesis.
In 1932, James Chadwick performed a series of experiments at the University of Cambridge, showing that the gamma ray hypothesis was untenable.[12] He suggested that the new radiation consisted of uncharged particles of approximately the mass of the proton, and he performed a series of experiments verifying his suggestion.[13] These uncharged particles were called neutrons, apparently from the Latin root for neutral and the Greek ending -on (by imitation of electron and proton).[14]
The discovery of the neutron explained a puzzle involving the spin of the nitrogen-14 nucleus, which had been experimentally measured to be 1 ħ. It was known that atomic nuclei usually had about half as many positive charges than if they were composed completely of protons, and in existing models this was often explained by proposing that nuclei also contained some "nuclear electrons" to neutralize the excess charge. Thus, nitrogen-14 would be composed of 14 protons and 7 electrons to give it a charge of +7 but a mass of 14 atomic mass units. However, it was also known that both protons and electrons carried an intrinsic spin of 1⁄2 ħ, and there was no way to arrange an odd number (21) of spins ±1⁄2 ħ to give a spin of 1 ħ. Instead, when nitrogen-14 was proposed to consist of 3 pairs of protons and neutrons, with an additional unpaired neutron and proton each contributing a spin of 1⁄2 ħ in the same direction for a total spin of 1 ħ, the model became viable. Soon, nuclear neutrons were used to naturally explain spin differences in many different nuclides in the same way, and the neutron as a basic structural unit of atomic nuclei was accepted.
Intrinsic properties
Stability and beta decay
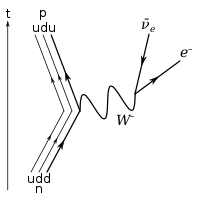
Under the Standard Model of particle physics, because the neutron consists of three quarks, the only possible decay mode without a change of baryon number is for one of the quarks to change flavour via the weak interaction. The neutron consists of two down quarks with charge −1⁄3 e and one up quark with charge +2⁄3 e, and the decay of one of the down quarks into a lighter up quark can be achieved by the emission of a W boson. By this means the neutron decays into a proton (which contains one down and two up quarks), an electron, and an electron antineutrino.
Free neutron decay
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 881.5±1.5 s (about 14 minutes, 42 seconds); therefore the half-life for this process (which differs from the mean lifetime by a factor of ln(2) = 0.693) is 611.0±1.0 s (about 10 minutes, 11 seconds).[5] Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as beta decay:[15]
n0
→
p+
+
e−
+
ν
e
A small fraction (about 1 in 1000) of free neutrons decay with the same products, but add an extra particle in the form of an emitted gamma ray:
n0
→
p+
+
e−
+
ν
e +
γ
This gamma ray may be thought of as a sort of "internal bremsstrahlung" which arises as the emitted beta particle interacts with the charge of the proton in an electromagnetic way.
Finally, a very small minority of neutron decays (about 4 per million) are so-called "two-body decays" in which the proton, electron and antineutrino are produced, but the electron fails to gain the 13.6 eV necessary energy to escape the proton, and therefore simply remains bound to it, as a neutral hydrogen atom. In this type of free neutron decay, essentially all of the decay energy is carried off by the antineutrino.
Bound neutron decay
Neutrons in unstable nuclei can also decay in the most common manner above. However, inside a nucleus, protons can also transform into a neutron via inverse beta decay. This transformation occurs by emission of an antielectron (also called positron) and an electron neutrino:
p+
→
n0
+
e+
+
ν
e
The transformation of a proton to a neutron inside of a nucleus is also possible through electron capture:
p+
+
e−
→
n0
+
ν
e
Positron capture by neutrons in nuclei that contain an excess of neutrons is also possible, but is hindered because positrons are repelled by the nucleus, and quickly annihilate when they encounter electrons.
When bound inside of a nucleus, the instability of a single neutron to beta decay is balanced against the instability that would be acquired by the nucleus as a whole if an additional proton were to participate in repulsive interactions with the other protons that are already present in the nucleus[clarification needed]. As such, although free neutrons are unstable, bound neutrons are not necessarily so. The same reasoning explains why protons, which are stable in empty space, may transform into neutrons when bound inside of a nucleus.
Electric dipole moment
The Standard Model of particle physics predicts a tiny separation of positive and negative charge within the neutron leading to a permanent electric dipole moment.[16] The predicted value is, however, well below the current sensitivity of experiments. From several unsolved puzzles in particle physics, it is clear that the Standard Model is not the final and full description of all particles and their interactions. New theories going beyond the Standard Model generally lead to much larger predictions for the electric dipole moment of the neutron. Currently, there are at least four experiments trying to measure for the first time a finite neutron electric dipole moment, including:
- Cryogenic neutron EDM experiment being set up at the Institut Laue–Langevin[17]
- nEDM experiment under construction at the new UCN source at the Paul Scherrer Institute[18]
- nEDM experiment being envisaged at the Spallation Neutron Source[19]
- nEDM experiment being built at the Institut Laue–Langevin[20]
Magnetic moment
Even though the neutron is a neutral particle, the magnetic moment of a neutron is not zero because it is a composite particle containing three charged quarks.
Anti-neutron
The antineutron is the antiparticle of the neutron. It was discovered by Bruce Cork in the year 1956, a year after the antiproton was discovered. CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles, so studying antineutrons yields provide stringent tests on CPT-symmetry. The fractional difference in the masses of the neutron and antineutron is (9±6)×10−5. Since the difference is only about two standard deviations away from zero, this does not give any convincing evidence of CPT-violation.[5]
Structure and geometry of charge distribution within the neutron
An article published in 2007 featuring a model-independent analysis concluded that the neutron has a negatively charged exterior, a positively charged middle, and a negative core.[21] In a simplified classical view, the negative "skin" of the neutron assists it to be attracted to the protons with which it interacts in the nucleus. However, the main attraction between neutrons and protons is via the nuclear force, which does not involve charge.
Neutron compounds
Dineutrons and tetraneutrons
The existence of stable clusters of 4 neutrons, or tetraneutrons, has been hypothesised by a team led by Francisco-Miguel Marqués at the CNRS Laboratory for Nuclear Physics based on observations of the disintegration of beryllium-14 nuclei. This is particularly interesting because current theory suggests that these clusters should not be stable.
The dineutron is another hypothetical particle.
Neutronium and neutron stars
At extremely high pressures and temperatures, nucleons and electrons are believed to collapse into bulk neutronic matter, called neutronium. This is presumed to happen in neutron stars.
The extreme pressure inside a neutron star may deform the neutrons into a cubic symmetry, allowing tighter packing of neutrons.[22]
Detection
The common means of detecting a charged particle by looking for a track of ionization (such as in a cloud chamber) does not work for neutrons directly. Neutrons that elastically scatter off atoms can create an ionization track that is detectable, but the experiments are not as simple to carry out; other means for detecting neutrons, consisting of allowing them to interact with atomic nuclei, are more commonly used. The commonly used methods to detect neutrons can therefore be categorized according to the nuclear processes relied upon, mainly neutron capture or elastic scattering. A good discussion on neutron detection is found in chapter 14 of the book Radiation Detection and Measurement by Glenn F. Knoll (John Wiley & Sons, 1979).
Neutron detection by neutron capture
A common method for detecting neutrons involves converting the energy released from neutron capture reactions into electrical signals. Certain nuclides have a high neutron capture cross section, which is the probability of absorbing a neutron. Upon neutron capture, the compound nucleus emits more easily detectable radiation, for example an alpha particle, which is then detected. The nuclides 3
He
, 6
Li
, 10
B
, 233
U
, 235
U
, 237
Np
and 239
Pu
are useful for this purpose.
Neutron detection by elastic scattering
Neutrons can elastically scatter off nuclei, causing the struck nucleus to recoil. Kinematically, a neutron can transfer more energy to light nuclei such as hydrogen or helium than to heavier nuclei. Detectors relying on elastic scattering are called fast neutron detectors. Recoiling nuclei can ionize and excite further atoms through collisions. Charge and/or scintillation light produced in this way can be collected to produce a detected signal. A major challenge in fast neutron detection is discerning such signals from erroneous signals produced by gamma radiation in the same detector.
Fast neutron detectors have the advantage of not requiring a moderator, and therefore being capable of measuring the neutron's energy, time of arrival, and in certain cases direction of incidence.
Uses
| Science with neutrons |
|---|
 |
| Foundations |
| Neutron scattering |
| Other applications |
|
| Infrastructure |
|
| Neutron facilities |
The neutron plays an important role in many nuclear reactions. For example, neutron capture often results in neutron activation, inducing radioactivity. In particular, knowledge of neutrons and their behavior has been important in the development of nuclear reactors and nuclear weapons. The fissioning of elements like uranium-235 and plutonium-239 is caused by their absorption of neutrons.
Cold, thermal and hot neutron radiation is commonly employed in neutron scattering facilities, where the radiation is used in a similar way one uses X-rays for the analysis of condensed matter. Neutrons are complementary to the latter in terms of atomic contrasts by different scattering cross sections; sensitivity to magnetism; energy range for inelastic neutron spectroscopy; and deep penetration into matter.
The development of "neutron lenses" based on total internal reflection within hollow glass capillary tubes or by reflection from dimpled aluminum plates has driven ongoing research into neutron microscopy and neutron/gamma ray tomography.[23][24][25]
A major use of neutrons is to excite delayed and prompt gamma rays from elements in materials. This forms the basis of neutron activation analysis (NAA) and prompt gamma neutron activation analysis (PGNAA). NAA is most often used to analyze small samples of materials in a nuclear reactor whilst PGNAA is most often used to analyze subterranean rocks around bore holes and industrial bulk materials on conveyor belts.
Another use of neutron emitters is the detection of light nuclei, particularly the hydrogen found in water molecules. When a fast neutron collides with a light nucleus, it loses a large fraction of its energy. By measuring the rate at which slow neutrons return to the probe after reflecting off of hydrogen nuclei, a neutron probe may determine the water content in soil.
Sources
Because free neutrons are unstable, they can be obtained only from nuclear disintegrations, nuclear reactions, and high-energy reactions (such as in cosmic radiation showers or accelerator collisions). Free neutron beams are obtained from neutron sources by neutron transport. For access to intense neutron sources, researchers must go to a specialist neutron facility that operates a research reactor or a spallation source.
The neutron's lack of total electric charge makes it difficult to steer or accelerate them. Charged particles can be accelerated, decelerated, or deflected by electric or magnetic fields. These methods have little effect on neutrons beyond a small effect of an inhomogeneous magnetic field because of the neutron's magnetic moment. Neutrons can be controlled by methods that include moderation, reflection and velocity selection.
Protection
Exposure to free neutrons can be hazardous, since the interaction of neutrons with molecules in the body can cause disruption to molecules and atoms, and can also cause reactions which give rise to other forms of radiation (such as protons). The normal precautions of radiation protection apply: avoid exposure, stay as far from the source as possible, and keep exposure time to a minimum. Some particular thought must be given to how to protect from neutron exposure, however. For other types of radiation, e.g. alpha particles, beta particles, or gamma rays, material of a high atomic number and with high density make for good shielding; frequently lead is used. However, this approach will not work with neutrons, since the absorption of neutrons does not increase straightforwardly with atomic number, as it does with alpha, beta, and gamma radiation. Instead one needs to look at the particular interactions neutrons have with matter (see the section on detection above). For example, hydrogen-rich materials are often used to shield against neutrons, since ordinary hydrogen both scatters and slows neutrons. This often means that simple concrete blocks or even paraffin-loaded plastic blocks afford better protection from neutrons than do far more dense materials. After slowing, neutrons may then be absorbed with an isotope which has high affinity for slow neutrons without causing secondary capture-radiation, such as lithium-6.
Hydrogen-rich ordinary water affects neutron absorption in nuclear fission reactors: usually neutrons are so strongly absorbed by normal water that fuel-enrichment with fissionable isotope is required. The deuterium in heavy water has a very much lower absorption affinity for neutrons than does protium (normal light hydrogen). Deuterium is therefore used in CANDU-type reactors, in order to slow (moderate) neutron velocity, to increase the probability of nuclear fission compared to neutron capture.
Production

Various nuclides become more stable by expelling neutrons as a decay mode; this is known as neutron emission, and happens commonly during spontaneous fission.
Cosmic radiation interacting with the Earth's atmosphere continuously generates neutrons that can be detected at the surface. Even stronger neutron radiation is produced at the surface of Mars where the atmosphere is thick enough to generate neutrons from cosmic ray spallation, but not thick enough to provide significant protection from the neutrons produced. These neutrons not only produce a Martian surface neutron radiation hazard from direct downward-going neutron radiation, but also a significant hazard from reflection of neutrons from the Martian surface, which will produce reflected neutron radiation penetrating upward into a Martian craft or habitat from the floor.[26]
Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing chain reaction. The intense neutron radiation can also be used to produce various radioisotopes through the process of neutron activation, which is a type of neutron capture.
Experimental nuclear fusion reactors produce free neutrons as a waste product. However, it is these neutrons that possess most of the energy, and converting that energy to a useful form has proved a difficult engineering challenge. Fusion reactors which generate neutrons are likely to create radioactive waste, but the waste is composed of neutron-activated lighter isotopes, which have relatively short (50–100 years) decay periods as compared to typical half lives of 10,000 years[citation needed] for fission waste, which is long primarily due to the long half life of alpha-emitting transuranic actinides.[27]
Neutron temperature
Thermal neutrons
A thermal neutron is a free neutron that is Boltzmann distributed with kT = 0.0253 eV (4.0×10−21 J) at room temperature. This gives characteristic (not average, or median) speed of 2.2 km/s. The name 'thermal' comes from their energy being that of the room temperature gas or material they are permeating. (see kinetic theory for energies and speeds of molecules). After a number of collisions (often in the range of 10–20) with nuclei, neutrons arrive at this energy level, provided that they are not absorbed.
In many substances, thermal neutrons have a much larger effective cross-section than faster neutrons, and can therefore be absorbed more easily by any atomic nuclei that they collide with, creating a heavier — and often unstable — isotope of the chemical element as a result.
Most fission reactors use a neutron moderator to slow down, or thermalize the neutrons that are emitted by nuclear fission so that they are more easily captured, causing further fission. Others, called fast breeder reactors, use fission energy neutrons directly.
Cold neutrons
Cold neutrons are thermal neutrons that have been equilibrated in a very cold substance such as liquid deuterium. Such a cold source is placed in the moderator of a research reactor or spallation source. Cold neutrons are particularly valuable for neutron scattering experiments.[citation needed]
Ultracold neutrons
Ultracold neutrons are produced by inelastically scattering cold neutrons in substances with a temperature of a few kelvins, such as solid deuterium or superfluid helium. An alternative production method is the mechanical deceleration of cold neutrons.
Fission energy neutrons
A fast neutron is a free neutron with a kinetic energy level close to 2 MeV (3.2×10−13 J), hence a speed of ~20000 km/s (~ 6% of the speed of light). They are named fission energy or fast neutrons to distinguish them from lower-energy thermal neutrons, and high-energy neutrons produced in cosmic showers or accelerators. Fast neutrons are produced by nuclear processes such as nuclear fission.
Fast neutrons can be made into thermal neutrons via a process called moderation. This is done with a neutron moderator. In reactors, typically heavy water, light water, or graphite are used to moderate neutrons.
Fusion neutrons
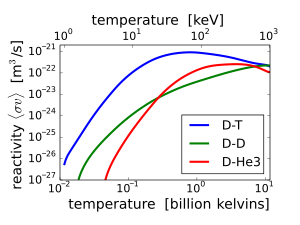
D-T (deuterium-tritium) fusion is the fusion reaction that produces the most energetic neutrons, with 14.1 MeV of kinetic energy and traveling at 17% of the speed of light. D-T fusion is also the easiest fusion reaction to ignite, reaching near-peak rates even when the deuterium and tritium nuclei have only a thousandth as much kinetic energy as the 14.1 MeV that will be produced.
14.1 MeV neutrons have about 10 times as much energy as fission neutrons, and are very effective at fissioning even non-fissile heavy nuclei, and these high-energy fissions produce more neutrons on average than fissions by lower-energy neutrons. This makes D-T fusion neutron sources such as proposed tokamak power reactors useful for transmutation of transuranic waste. 14.1 MeV neutrons can also produce neutrons by knocking them loose from nuclei.
On the other hand, these very high energy neutrons are less likely to simply be captured without causing fission or spallation. For these reasons, nuclear weapon design extensively utilizes D-T fusion 14.1 MeV neutrons to cause more fission. Fusion neutrons are able to cause fission in ordinarily non-fissile materials, such as depleted uranium (uranium-238), and these materials have been used in the jackets of thermonuclear weapons. Fusion neutrons also can cause fission in substances that are unsuitable or difficult to make into primary fission bombs, such as reactor grade plutonium. This physical fact thus causes ordinary non-weapons grade materials to become of concern in certain nuclear proliferation discussions and treaties.
Other fusion reactions produce much less energetic neutrons. D-D fusion produces a 2.45 MeV neutron and helium-3 half of the time, and produces tritium and a proton but no neutron the other half of the time. D-3He fusion produces no neutron.
Intermediate-energy neutrons
A fission energy neutron that has slowed down but not yet reached thermal energies is called an epithermal neutron.
Cross sections for both capture and fission reactions often have multiple resonance peaks at specific energies in the epithermal energy range. These are of less significance in a fast neutron reactor where most neutrons are absorbed before slowing down to this range, or in a well-moderated thermal reactor where epithermal neutrons mostly interact with moderator nuclei, not with either fissile or fertile actinide nuclides. However, in a partially moderated reactor with more interactions of epithermal neutrons with heavy metal nuclei, there are greater possibilities for transient changes in reactivity which might make reactor control more difficult.
Ratios of capture reactions to fission reactions are also worse (more captures without fission) in most nuclear fuels such as plutonium-239, making epithermal-spectrum reactors using these fuels less desirable, as captures not only waste the one neutron captured but also usually result in a nuclide which is not fissile with thermal or epithermal neutrons, though still fissionable with fast neutrons. The exception is uranium-233 of the thorium cycle which has good capture-fission ratios at all neutron energies.
High-energy neutrons
These neutrons have more energy than fission energy neutrons and are generated as secondary particles by particle accelerators or in the atmosphere from cosmic rays. They can have energies as high as tens of joules per neutron. These neutrons are extremely efficient at ionization and far more likely to cause cell death than X-rays or protons.[28][29]
See also
- Ionizing radiation
- Isotope
- List of particles
- Neutron capture nucleosynthesis
- Neutron magnetic moment
- Neutron radiation and the Sievert radiation scale
- Nuclear reaction
- Thermal reactor
Neutron sources
Processes involving neutrons
References
- ^ a b 1935 Nobel Prize in Physics
- ^ a b http://chemed.chem.purdue.edu/genchem/history/rutherford.html
- ^ a b c d e f P.J. Mohr, B.N. Taylor, and D.B. Newell (2011), "The 2010 CODATA Recommended Values of the Fundamental Physical Constants" (Web Version 6.0). This database was developed by J. Baker, M. Douma, and S. Kotochigova. Available: http://physics.nist.gov/constants [Thursday, 02-Jun-2011 21:00:12 EDT]. National Institute of Standards and Technology, Gaithersburg, MD 20899.
- ^ http://ansnuclearcafe.org/2011/10/19/pioneers102011/
- ^ a b c K. Nakamura et al. (Particle Data Group), JP G 37, 075021 (2010) and 2011 partial update for the 2012 edition
- ^ Nudat 2. Nndc.bnl.gov. Retrieved on 2010-12-04.
- ^ Rutherford, E. (1920). "Bakerian Lecture. Nuclear Constitution of Atoms". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 97 (686): 374. doi:10.1098/rspa.1920.0040. JSTOR 93888.
- ^ "V. A. Ambartsumian— a life in science" (PDF). Astrophysics. 51 (3): 280. 2008. Bibcode:2008Ap.....51..280T. doi:10.1007/s10511-008-9016-6.
- ^ Bothe, W.; Becker, H. (1930). "K�nstliche Erregung von Kern-?-Strahlen". Zeitschrift f�r Physik. 66 (5–6): 289. doi:10.1007/BF01390908.
{{cite journal}}: replacement character in|journal=at position 14 (help); replacement character in|title=at position 2 (help) - ^ Becker, H.; Bothe, W. (1932). "Die in Bor und Beryllium erregten ?-Strahlen". Zeitschrift f�r Physik. 76 (7–8): 421. doi:10.1007/BF01336726.
{{cite journal}}: replacement character in|journal=at position 14 (help) - ^ "Émission de protons de grande vitesse par les substances hydrogénées sous l'influence des rayons g très pénétrants".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Chadwick, J. (1933). "Bakerian Lecture. The Neutron". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 142 (846): 1. doi:10.1098/rspa.1933.0152.
- ^ Chadwick, James (1932). "Possible Existence of a Neutron". Nature. 129 (3252): 312. Bibcode:1932Natur.129Q.312C. doi:10.1038/129312a0.
- ^ "Wolfgang Pauli". Sources in the History of Mathematics and Physical Sciences. 6. 1985: 105. doi:10.1007/978-3-540-78801-0_3. ISBN 978-3-540-13609-5.
{{cite journal}}:|chapter=ignored (help); Cite journal requires|journal=(help) - ^ Particle Data Group Summary Data Table on Baryons
- ^ "Pear-shaped particles probe big-bang mystery" (Press release). University of Sussex. 20 February 2006. Retrieved 2009-12-14.
- ^ A cryogenic experiment to search for the EDM of the neutron
- ^ Search for the neutron electric dipole moment: nEDM
- ^ SNS Neutron EDM Experiment
- ^ Measurement of the Neutron Electric Dipole Moment
- ^ G.A. Miller (2007). "Charge Densities of the Neutron and Proton". Physical Review Letters. 99 (11): 112001. Bibcode:2007PhRvL..99k2001M. doi:10.1103/PhysRevLett.99.112001.
- ^ Felipe J. Llanes-Estrada, Gaspar Moreno Navarro., Felipe J.; Gaspar Moreno Navarro (2011). "Cubic neutrons". arXiv:1108.1859 [nucl-th].
{{cite arXiv}}: Unknown parameter|version=ignored (help) - ^ Kumakhov, M. A. (1992). "A neutron lens". Nature. 357 (6377): 390–391. Bibcode:1992Natur.357..390K. doi:10.1038/357390a0.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Physorg.com, "New Way of 'Seeing': A 'Neutron Microscope'"
- ^ NASA.gov: "NASA Develops a Nugget to Search for Life in Space"
- ^ Clowdsley, MS; Wilson, JW; Kim, MH; Singleterry, RC; Tripathi, RK; Heinbockel, JH; Badavi, FF; Shinn, JL (2001). "Neutron Environments on the Martian Surface" (PDF). Physica Medica. 17 (Suppl 1): 94–6. PMID 11770546.
- ^ Science/Nature | Q&A: Nuclear fusion reactor. BBC News (2006-02-06). Retrieved on 2010-12-04.
- ^ "Facing up to secondary neutrons". Medical Physics Web. May 23, 2008. Retrieved 2011-02-08.
{{cite web}}:|first=missing|last=(help) - ^ Heilbronn, L. Heilbronn (20 December 2005). "Expand+Overview of secondary neutron production relevant to shielding in space". Radiation Protection Dosimetry. 116 (1–4): 140–143. doi:10.1093/rpd/nci033. PMID 16604615. Retrieved 2011-02-08.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
Further reading
- Annotated bibliography for neutrons from the Alsos Digital Library for Nuclear Issues
- Knoll, G. F. (2000) Radiation Detection and Measurement
- Krane, K. S. (1998) Introductory Nuclear Physics
- Squires, G. L. (1997) Introduction to the Theory of Thermal Neutron Scattering
- Dewey, M. S., Gilliam, D. M., Nico, J. S., Snow, M. S., Wietfeldt, F. E. NIST Neutron Lifetime Experiment
