Diabetic nephropathy: Difference between revisions
→Pathophysiology: Fixed link for "ACE inhibitor" from "ACE inhibitor drugs" which did not have an associated Wikipedia page. |
Factsearch (talk | contribs) →Treatment: provided update |
||
| Line 119: | Line 119: | ||
[[C-peptide]], a by-product of insulin production, may provide new hope for patients suffering from diabetic nephropathy.<ref>{{cite journal |author=Wahren J, Ekberg K, Jörnvall H |title=C-peptide is a bioactive peptide |journal=Diabetologia |volume=50 |issue=3 |pages=503–9 |year=2007 |pmid=17235526 |doi=10.1007/s00125-006-0559-y }}</ref> |
[[C-peptide]], a by-product of insulin production, may provide new hope for patients suffering from diabetic nephropathy.<ref>{{cite journal |author=Wahren J, Ekberg K, Jörnvall H |title=C-peptide is a bioactive peptide |journal=Diabetologia |volume=50 |issue=3 |pages=503–9 |year=2007 |pmid=17235526 |doi=10.1007/s00125-006-0559-y }}</ref> |
||
In August 2014, [[AstraZeneca]] announced it had agreed to collaborate with [[Mitsubishi Tanabe Pharma]] to leverage the pair's strengths, expertise and assets on diabetic nephropathy, in a bid to develop high quality drugs much quicker than working alone. According to the [[National Institute of Health]] 60% to 70% of diabetic sufferers in the U.S. alone suffered from nerve disorders related to diabetic nephropathy. The three year research agreement had the objective of creating new treatments to replace expensive and limited options currently in place, mainly being dialysis or kidney transplantation.<ref name="AstraZenecaMTPC">{{cite news|title=AstraZeneca and MTPC come together for research on diabetic nephropathy drugs|url=http://www.businesssun.com/index.php/sid/224938259|date=20 August 2014|accessdate=21 August 2014|publisher=''Business Sun''}}</ref> |
|||
===Compounds in Development=== |
===Compounds in Development=== |
||
Revision as of 23:52, 21 August 2014
This article needs additional citations for verification. (November 2011) |
| Diabetic nephropathy | |
|---|---|
| Specialty | Nephrology, endocrinology |
Diabetic nephropathy (nephropatia diabetica), also known as Kimmelstiel–Wilson syndrome, or nodular diabetic glomerulosclerosis[1] and intercapillary glomerulonephritis, is a progressive kidney disease caused by angiopathy of capillaries in the kidney glomeruli. It is characterized by nephrotic syndrome and diffuse glomerulosclerosis. It is due to longstanding diabetes mellitus, and is a prime indication for dialysis in many developed countries. It is classified as a microvascular complication of diabetes.[2]
Signs and symptoms
Kidney failure provoked by glomerulosclerosis leads to fluid filtration deficits and other disorders of kidney function. There is an increase in blood pressure (hypertension) and fluid retention in the body plus a reduced plasma oncotic pressure causing edema. Other complications may be arteriosclerosis of the renal artery and proteinuria.
Throughout its early course, diabetic nephropathy has no symptoms. They develop in late stages and may be a result of excretion of high amounts of protein in the urine or due to renal failure:
- edema: swelling, usually around the eyes in the mornings; later, general body swelling may result, such as swelling of the legs
- foamy appearance or excessive frothing of the urine (caused by the proteinuria)
- unintentional weight gain (from fluid accumulation)
- anorexia (poor appetite)
- nausea and vomiting
- malaise (general ill feeling)
- fatigue
- headache
- frequent hiccups
The first laboratory abnormality is a positive microalbuminuria test. Most often, the diagnosis is suspected when a routine urinalysis of a person with diabetes shows too much protein in the urine (proteinuria). The urinalysis may also show glucose in the urine, especially if blood glucose is poorly controlled. Serum creatinine and BUN may increase as kidney damage progresses.
A kidney biopsy confirms the diagnosis, although it is not always necessary if the case is straightforward, with a documented progression of proteinuria over time and presence of diabetic retinopathy on examination of the retina of the eyes.
Cause
The word diabetes means "passing through", referring to the polyuria (abnormal increase of urine production), a symptom historically present in those affected by the disease. When the level of blood glucose rises beyond the kidney's capacity to reabsorb glucose from the renal ultrafiltrate, glucose remains diluted in the fluid, raising its osmotic pressure and causing more water to be carried out, thus, increasing the excreted urine volume. The increased volume dilutes the sodium chloride in the urine, signalling the macula densa to release more renin, causing vasoconstriction, a survival mechanism to retain water by passing less blood through the kidneys. Because the kidney is nurtured exclusively by the blood it filtrates, the vasoconstriction also reduces the nutrients supplied to it, causing infarct of its tissues and reduction of renal function.
Pathophysiology

Glomerular Hyperfiltration is the basic pathophysiology in Diabetic nephropathy. This leads to intraglomerular hypertension. ACE inhibitor drugs help prevent diabetic nephropathy by preventing this step. Progression from glomerular hyperfilteration leads to the stage of basement membrane thickening. This is the earliest detectable change in the course of diabetic nephropathy. This is followed by expansion of mesangium and finally by nodular sclerosis. At this stage, the kidney may leak more serum albumin(plasma protein) than normal in the urine(albuminuria), and this can be detected by sensitive medical tests for albumin. This stage is called "microalbuminuria". As diabetic nephropathy progresses, increasing numbers of glomeruli are destroyed by progressive nodular glomerulosclerosis. Consequently, urine albumin increases to the point that it may be detected by ordinary urinalysis techniques. At this stage, a kidney biopsy generally clearly shows diabetic nephropathy. The Armanni-Ebstein change or Armanni-Ebstein cells consists of deposits of glycogen in the tubular epithelial cells (pars straight of proximal convoluted tubule and loop of Henle). Because most diabetics are treated before this stage, it is very rare to see it at the present time. It appears in decompensated diabetics with glycemia higher than 500 mg/dL and in the presence of severe glycosuria; it is a reversible alteration without functional manifestations. The interstitium shows nonspecific chronic changes.
Diagnosis
Diagnosis is based on the measurement of urinary albumin. We can define:
- Normoalbuminuria: urinary albumin excretion <30 mg/24h, it is the physiological state;
- Microalbuminuria: urinary albumin excretion in the range of 30–299 mg/24h;
- Clinical (overt) albuminuria: urinary albumin excretion ≥300 mg/24h.
Diabetic patients are suggested to control albumin excretion every year. Urinary albumin collection can also be timed (normal value <20 mg/min) or a random spot collection (normal value <30 μg/mg). Abnormal values correlate with nephropathy.
| CKD Stage | eGFR level (mL/min/1.73 m2) |
|---|---|
| Stage 1 | ≥ 90 |
| Stage 2 | 60 – 89 |
| Stage 3 | 30 – 59 |
| Stage 4 | 15 – 29 |
| Stage 5 | < 15 |
Another diagnostic tool is glomerular filtration rate esteem (eGFR) based on Cockroft and Gault or on Levey’s (MDRD modified) formulae, both based on creatinine values and patient’s age. Normal eGFR is above 90 mm/min/1.73 m2; different stages of renal damage can be identified by eGFR intervals. Before the use of eGFR, GFR was calculated using invasive technique such as inulin (or radioactive inulin analogues) injection.
Diabetic nephropathy is usually preceded by the onset of diabetic retinopathy; the evidence of nephropathy without retinopathy gives the suspicion that the renal impairment is not caused by diabetes itself but it is the result of comorbidity (e.g. glomerulonephritis).
| Stage | Designation | Characteristics | Structural Changes | Glomerular Filtration Rate mL/min 1.73 m2 | Blood Pressure mm Hg |
|---|---|---|---|---|---|
| I | Hyperfunction | Hyperfiltration | Glomerular hypertrophy | >150 | Normal |
| II | Normoalbuminuria | Normal albumin loss | Basement membrane thickening | 150 | Normal |
| III | Incipient diabetic nephropathy (microalbuminuria) | Increased albumin loss | Albumin loss correlates with structural damage and hypertrophy of remaining glomeruli | 125 | Increased |
| IV | Overt diabetic nephropathy | Clinical proteinuria | Advanced structural damage | <100 | Hypertension |
| V | Uremia | Kidney failure | Glomerular closure | 0-10 | High |
Treatment
The goals of treatment are to slow the progression of kidney damage and control related complications. The main treatment, once proteinuria is established, is ACE inhibitor drugs, which usually reduces proteinuria levels and slows the progression of diabetic nephropathy. Several effects of the ACEIs that may contribute to renal protection have been related to the association of rise in Kinins which is also responsible for some of the side effects associated with ACEIs therapy such as dry cough. The renal protection effect is related to the antihypertensive effects in normal and hypertensive patients, renal vasodilatation resulting in increased renal blood flow and dilatation of the efferent arterioles.[4] Many studies have shown that related drugs, angiotensin receptor blockers (ARBs), have a similar benefit. However, combination therapy, according to the ONTARGET study,[5] is known to worsen major renal outcomes, such as increasing serum creatinine and causing a greater decline in estimated glomerular filtration rate (eGFR).
Blood-glucose levels should be closely monitored and controlled. This may slow the progression of the disorder, especially in the very early ("microalbuminuria") stages. Medications to manage diabetes include oral hypoglycemic agents and insulin injections. As kidney failure progresses, less insulin is excreted, so lesser doses may be needed to control glucose levels.
Diet may be modified to help control blood-sugar levels.[5] Modification of protein intake can affect hemodynamic and nonhemodynamic injury.
High blood pressure should be aggressively treated with antihypertensive medications, in order to reduce the risks of kidney, eye, and blood vessel damage in the body. It is also very important to control lipid levels, maintain a healthy weight, and engage in regular physical activity.
Patients with diabetic nephropathy should avoid taking the following drugs:
- Contrast agents containing iodine
- Commonly used non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen and naproxen, or COX-2 inhibitors like celecoxib, because they may injure the weakened kidney.
Urinary tract and other infections are common and can be treated with appropriate antibiotics.
Dialysis may be necessary once end-stage renal disease develops. At this stage, a kidney transplantation must be considered. Another option for type 1 diabetes patients is a combined kidney-pancreas transplant.
C-peptide, a by-product of insulin production, may provide new hope for patients suffering from diabetic nephropathy.[6]
In August 2014, AstraZeneca announced it had agreed to collaborate with Mitsubishi Tanabe Pharma to leverage the pair's strengths, expertise and assets on diabetic nephropathy, in a bid to develop high quality drugs much quicker than working alone. According to the National Institute of Health 60% to 70% of diabetic sufferers in the U.S. alone suffered from nerve disorders related to diabetic nephropathy. The three year research agreement had the objective of creating new treatments to replace expensive and limited options currently in place, mainly being dialysis or kidney transplantation.[7]
Compounds in Development
Several compounds are in development for diabetic kidney disease. These include, but are not limited to, bardoxolone methyl,[8] olmesartan medoxomil, sulodexide, NOX-E36,[9] and avosentan.[10]
Prognosis
Diabetic nephropathy continues to get gradually worse. Complications of chronic kidney failure are more likely to occur earlier, and progress more rapidly, when it is caused by diabetes than other causes. Even after initiation of dialysis or after transplantation, people with diabetes tend to do worse than those without diabetes.
Possible complications include:
- hypoglycemia (due to decreased renal clearance of insulin)
- rapidly progressing chronic kidney failure
- end-stage kidney disease
- hyperkalemia
- severe hypertension
- complications of hemodialysis
- complications of kidney transplant
- coexistence of other diabetes complications
- peritonitis (if peritoneal dialysis used)
- increased infections
Epidemiology
The syndrome can be seen in patients with diabetes (usually less than 15 years after onset) after about 5 years in type 1 diabetes. Clinical nephropathy secondary to glomerular disease usually manifests 15–25 years after diagnosis of diabetes and affects 25-35% of patients under the age of 30 years. It is the leading cause of premature death in young diabetic patients (between 50 and 70 years old). The disease is progressive and may cause death two or three years after the initial lesions, and is more frequent in men. Diabetic nephropathy is the most common cause of chronic kidney failure and end-stage kidney disease in the United States. People with both type 1 and type 2 diabetes are at risk. The risk is higher if blood-glucose levels are poorly controlled. Furthermore, once nephropathy develops, the greatest rate of progression is seen in patients with poor control of their blood pressure. Also people with high cholesterol level in their blood have much more risk than others.
History
The syndrome was discovered by British physician Clifford Wilson (1906–1997) and German-born American physician Paul Kimmelstiel (1900–1970) and was published for the first time in 1936.[11]
See also
Additional images
-
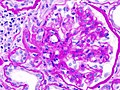
Histopathological image of diabetic glomerulosclerosis with nephrotic syndrome. H&E stain.
-
Histopathological image of diabetic glomerulosclerosis with nephrotic syndrome. Another glomerulus. H&E stain.
-
Histopathological image of diabetic glomerulosclerosis with nephrotic syndrome. Another glomerulus. H&E stain.
-
Histopathological image of diabetic glomerulosclerosis with nephrotic syndrome. PAS stain.
-
Histopathological image of diabetic glomerulosclerosis with nephrotic syndrome. PAS stain.
-
Histopathological image of diabetic glomerulosclerosis with nephrotic syndrome. PAM stain.
-
Histopathological image of diabetic glomerulosclerosis with nephrotic syndrome. PAM stain.
References
- ^ Berkman, James; Rifkin, Harold (1973). "Unilateral nodular diabetic glomerulosclerosis (Kimmelstiel–Wilson): Report of a case". Metabolism. 22 (5): 715–722. doi:10.1016/0026-0495(73)90243-6. PMID 4704716.
- ^ Longo et al., Harrison's Principles of Internal Medicine, 18th ed., p.2982
- ^ Burtis, C.A.; Ashwood, E.R. and Bruns, D.E. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th Edition. Elsevier Saunders. p.1560
- ^ Diabetes Mellitus and Angiotensin Converting Enzyme Inhibitors
- ^ a b The ONTARGET Investigators; Yusuf, S; Teo, KK; Pogue, J; Dyal, L; Copland, I; Schumacher, H; Dagenais, G; Sleight, P (2008). "Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events". New England Journal of Medicine. 358 (15): 1547–59. doi:10.1056/NEJMoa0801317. PMID 18378520.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) Cite error: The named reference "dtdn" was defined multiple times with different content (see the help page). - ^ Wahren J, Ekberg K, Jörnvall H (2007). "C-peptide is a bioactive peptide". Diabetologia. 50 (3): 503–9. doi:10.1007/s00125-006-0559-y. PMID 17235526.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "AstraZeneca and MTPC come together for research on diabetic nephropathy drugs". Business Sun. 20 August 2014. Retrieved 21 August 2014.
{{cite news}}: Italic or bold markup not allowed in:|publisher=(help) - ^ http://www.medscape.com/viewarticle/590644
- ^ Clinical trial number NCT01547897 for "NOX-E36 in Patients With Type 2 Diabetes Mellitus and Albuminuria" at ClinicalTrials.gov
- ^ http://www.medicalnewstoday.com/articles/139028.php
- ^ Kimmelstiel P, Wilson C (1936). "Benign and malignant hypertension and nephrosclerosis. A clinical and pathological study". Am J Pathol. 12 (1): 45–48. PMC 1911030. PMID 19970253.
Berlowitz, D. R. and Weinberg, M. H. (1998). In adequate management of blood glucose in diabetic population. N England J. Med. 339: 1957 – 1963. Burt, V. L., R. and Duston, H. P. (1995). Prevalence of hypertension in the adult US population: Results from the third National Health and Nutrition examination survey (1988 - 1991) 25:305 – 313. Ferro, P. V. and Ham., A. B. (1957). American journal on clinical pathology 28:208 – 211. http://www.WHO.org. (2012). Therapic Review. Patient with diabetes nephropathy-medication for urinary tract infection. Israli, Z. H (1992). Cough and Angiotensin Converting Enzyme Inhibitor therapy, a review of literature and pathophisiology. Ann Inter. Med. 117:234 – 242. Nice, C. K. S. (2010). Clinical guidelines on diabetes mellitus type II. Shaid, S., Akram, H., Jaeed, M. and Mahbood, T. (2004) similar nature of ionic imbalance in cardiovascular and renal disorders, Pakistan Journal of medicine 4th edition, Oxford University Press. Vol. 2, 15:1165 – 1198. Whitby, L. G., Percy – Robb, I. W. and Smith, A. F. (1984). Lecture notes on clinical chemistry 3rd edition, Blackwell scientific publication, Oxford 20:301 – 304. White, W. L. Skeggs, L. T. and Hochstrasser, H. C. (1970). Chemistry of technologist, 3rd edition C. V. Mosby co. st. Louise, 4:182 – 183. William, G. H., Weir, M. R. and Ruddly, I. B. (1988). Converting enzyme inhibitors in the treatment of diabetes mellitus. N. Engl. Journal on medicine, 319: 174 – 179. Zelmanovitz, T., Grechman, F., Balthazar, A. P. (2009). Diabetic nephropathy. Diabetol metab synd. 21:1 – 10.
External links
- Diabetic nephropathy. HealthCentral.
- Diabetic nephropathy. MedlinePlus Medical Encyclopedia. Text from this public domain article was partially used here.
- Texas University Classification
This template is no longer used; please see Template:Endocrine pathology for a suitable replacement