Ctenophora: Difference between revisions
→Ecology: ''Lampea'' |
→Lobates: emergency jet propulsion |
||
| Line 167: | Line 167: | ||
===Lobates=== |
===Lobates=== |
||
[[Image:Bathocyroe fosteri.jpg|thumb|right|150px|''Bathocyroe fosteri'' a common but fragile deep-sea lobate, oriented mouth down]] |
[[Image:Bathocyroe fosteri.jpg|thumb|right|150px|''Bathocyroe fosteri'' a common but fragile deep-sea lobate, oriented mouth down]] |
||
The [[Lobata]] have a pair of lobes, muscular extensions of the body that project ahead of the mouth. Their small tentacles run in convoluted grooves over the inner surface of the lobes. Between the lobes and surrounding the mouth are four auricles, projections fringed with cilia that produce water currents that wash microscopic prey into the mouth. This combination of structures enables lobates to feed continuously on suspended prey such as [[plankton]].<ref name="RuppertBarnes2004Ctenophora" /> There are eight comb-rows, two on each lobe and two on each side beteeen the lobes. Members of the [[genus | genera]] ''[[Leucothea]]'' and ''[[Ocyropsis]]'' also use their large lobes for swimming.<ref name="RuppertBarnes2004Ctenophora" /><ref name="RuppertBarnes2004Ctenophora" /> Unlike cydippids, the movements of lobates' combs are co-ordinated by nerves rather than by via water disturbances created by the cilia, and combs on the same row beat together rather than in [[Mexican wave]] style. This may have enabled lobates to grow larger than cydippids and to have shapes that are less egg-like.<ref name="CraigOkubo1990">{{cite journal |
The [[Lobata]] have a pair of lobes, muscular extensions of the body that project ahead of the mouth. Their small tentacles run in convoluted grooves over the inner surface of the lobes. Between the lobes and surrounding the mouth are four auricles, projections fringed with cilia that produce water currents that wash microscopic prey into the mouth. This combination of structures enables lobates to feed continuously on suspended prey such as [[plankton]].<ref name="RuppertBarnes2004Ctenophora" /> |
||
There are eight comb-rows, two on each lobe and two on each side beteeen the lobes. Members of the [[genus | genera]] ''[[Leucothea]]'' and ''[[Ocyropsis]]'' also use their large lobes for swimming.<ref name="RuppertBarnes2004Ctenophora" /><ref name="RuppertBarnes2004Ctenophora" /> Unlike cydippids, the movements of lobates' combs are co-ordinated by nerves rather than by via water disturbances created by the cilia, and combs on the same row beat together rather than in [[Mexican wave]] style. This may have enabled lobates to grow larger than cydippids and to have shapes that are less egg-like.<ref name="CraigOkubo1990">{{cite journal |
|||
| author=Craig, C.L., and and Okubo, A. | title=Physical constraints on the evolution of ctenophore size and shape |
| author=Craig, C.L., and and Okubo, A. | title=Physical constraints on the evolution of ctenophore size and shape |
||
| journal=Evolutionary Ecology | volume=4 | issue=2 | date=April 1990 | pages=115-129 | doi=10.1007/BF02270909 |
| journal=Evolutionary Ecology | volume=4 | issue=2 | date=April 1990 | pages=115-129 | doi=10.1007/BF02270909 |
||
}}</ref> In addition members of the lobate [[genus | genera]] ''[[Bathocyroe]]'' and ''[[Ocyropsis]]'' can escape from danger by clapping their lobes, so that the jet of expelled water drives them backwards very quickly.<ref name="Haddock2007ComparativeFeeding" /> |
|||
| ⚫ | |||
| ⚫ | |||
| author=Horita, T. | date=March 2000 |
| author=Horita, T. | date=March 2000 |
||
| title=An undescribed lobate ctenophore, ''Lobatolampea tetragona'' gen. nov. & spec. nov., representing a new family, from Japan |
| title=An undescribed lobate ctenophore, ''Lobatolampea tetragona'' gen. nov. & spec. nov., representing a new family, from Japan |
||
| Line 177: | Line 181: | ||
}}</ref> |
}}</ref> |
||
{{clear}} |
{{clear}} |
||
===Beroids=== |
===Beroids=== |
||
{{Annotated image | float=right | caption=A beroid ctenophore about to attack. The open mouth is on the left. This animal is 3-6 cm long. |
{{Annotated image | float=right | caption=A beroid ctenophore about to attack. The open mouth is on the left. This animal is 3-6 cm long. |
||
Revision as of 19:44, 12 February 2009
This article needs attention from an expert in Tree of Life. Please add a reason or a talk parameter to this template to explain the issue with the article. (November 2008) |
| Comb jellies | |
|---|---|

| |
| "Ctenophorae" from Ernst Haeckel's Kunstformen der Natur, 1904 | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | Ctenophora Eschscholtz, 1829
|
| Classes | |
The phylum Ctenophora (Template:PronEng), commonly known as comb jellies, is a phylum that includes the sea gooseberry (Pleurobrachia pileus) and Venus' girdle (Cestum veneris). Classically grouped with Cnidaria (jellyfish) in the Coelenterata infrakingdom, ctenophores have recently been identified as the most basal known lineage of animals.[1]
Despite their appearance, they are zoologically not jellyfish, not least because they lack the characteristic cnidocytes (stinging cells) but have connective tissues and a nervous system. There are close to 150 described species of ctenophora spread throughout the world's oceans, from shallow estuarine waters to the deep sea. Although there are a few benthic species, most are members of the gelatinous zooplankton and form a considerable proportion of the entire plankton biomass worldwide. A few species, such as the sea gooseberry, native to the North Sea, have reached such high populations that they clog fishermen's nets, while of other species only a few examples are known. The fragile makeup of ctenophores makes research into their way of life extremely difficult; for this reason data on their lifespan are not available, but it is known that ctenophores begin to reproduce at an early age and so can be assumed to have a short generation cycle.
The word ctenophore (Template:PronEng or /ˈtiːnəfɔər/, without the c) comes from Greek, kteno-, kteis, "comb" and -phore, meaning "bearer". It comes via the New Latin ctenophorus in the 19th century.
Distinguishing features
Ctenophores form an animal phylum that is more complex than sponges, about as complex as cnidarians (jellyfish, sea anemones, etc.), and less complex than bilaterians, which include almost all other animals. However both ctenophores and cnidarians are more complex than sponges as they have: cells bound by inter-cell connections and carpet-like basement membranes; muscles; nervous systems; and some have sensory organs. Ctenophores are distinguished from all other animals by having colloblasts that capture prey by squirting glue on them, although a few ctenophore species lack them.[2][3]
Ranging from about 1 millimetre (0.039 in) to 1.5 metres (4.9 ft) in size,[4][5] ctenophores are the largest non-colonial animals that use cilia ("hairs") as their main method of locomotion.[5] Most species have up to eight strips that run the length of their bodies and bear comb-like bands of cilia running at intervals across the strips.[5] The name "ctenophore" means "comb-bearing", from the Greek κτείς (stem-form κτεν-) meaning "comb" and the Greek suffix -φορος meaning "carrying".[6]
Like sponges and cnidarians, ctenophores have two main layers of cells that sandwich a middle layer of jelly-like material, which is called the mesoglea in cnidarians; more complex animals have three main cell layers and no intermediate jelly-like layer. Hence ctenophores and cnidarians have traditionally been labelled diploblastic, along with sponges.[2][7] However both ctenophores and cnidarians have a type of muscle which, in more complex animals, arises from the middle cell layer.[8] As a result some recent text books classify ctenophores as triploblastic,[5] while others disagree.[2]
| Sponges[9][10] | Cnidarians[2][7] | Ctenophores[2][5] | Bilateria[2] | |
|---|---|---|---|---|
| Cnidocytes | No | Yes | No | |
| Colloblasts | No | In most species[3] | No | |
| Digestive and circulatory organs | No | Yes | ||
| Number of main cell layers | Two, with jelly-like layer between them | Debate about whether two[2] or three[5][8] | three | |
| Cells in each layer bound together | No, except that Homoscleromorpha have basement membranes.[11] | Yes: inter-cell connections; basement membranes | ||
| Sensory organs | No | Yes | ||
| Number of cells in middle "jelly" layer | Many | Few | (not applicable) | |
| Cells in outer layers can move inwards and change functions | Yes | No | (not applicable) | |
| Nervous system | No | Yes, simple | Simple to complex | |
| Muscles | None | Mostly epitheliomuscular | Mostly myoepithelial | Mostly myocytes |
Description
For a phylum with relatively few species, ctenophores have a wide range of body plans.[5] Coastal species need to be tough enough to withstand waves and swirling sediment particles, while oceanic species are so fragile that it is very difficult to capture them intact for study.[3] In addition oceanic species do not preserve well,[3] and are known mainly from photographs and from observers' notes.[12] Hence most attention has until recently concentrated on three genera – Pleurobrachia, Beroe and Mnemiopsis.[13][3] At least two textbooks base their descriptions of ctenophores on the cydippid Pleurobrachia.[5][2]
Common features
Body layers
Like those of cnidarians, (jellyfish, sea anemones, etc.), ctenophores' bodies consist of a relatively thick, jelly-like mesoglea sandwiched between two epithelia, layers of cells bound by inter-cell connections and by a fibrous basement membrane which they secrete.[2][5] However the epithelia of ctenophores have two layers of cells rather than one, and the cells in the upper layer generally have several cilia per cell.[5]
The outer layer of the epidermis (outer skin) consists of: sensory cells; cells that secrete mucus, which protects the body; and interstitial cells, which can transform into other types of cell. In specialized parts of the body the outer layer also contains colloblasts, used in capturing prey, or cells bearing multiple large cilia, for locomotion. The inner layer of the epidermis contains a nerve net, and myoepithelial cells which act as muscles.[5]
The internal cavity forms: a mouth that can usually be closed by muscles; a pharynx ("throat"); a wider area in the center that acts as a stomach; and a system of internal canals. These branch through the mesoglea to the most active parts of the animal: the mouth and pharynx; the roots of the tentacles, if present; all along the underside of each comb row; and four branches round the sensory complex at the far end from the mouth – two of these four branches terminate in anal pores. The inner surface of the cavity is lined with an epithelium, the gastrodermis. The mouth and pharynx have both cilia and well-developed muscles. In other parts of the canal system, the gastrodermis is different on the sides nearest to and furthest from the organ that it supplies. The nearer side is composed of tall nutritive cells that store nutrients in vacuoles (internal compartments), germ cells that produce eggs or sperm, and photocytes that produce bioluminescence. The side furthest from the organ is covered with ciliated cells that circulate water through the canals, punctuated by ciliary rosettes, pores that are surrounded by double whorls of cilia and connect to the mesoglea.[5]
Feeding, excretion and respiration
When prey enters the mouth, it is liquidized in the pharynx by enzymes and by muscular contractions of the pharynx. The resulting slurry is wafted through the canal system by the beating of the cilia, and digested by the nutritive cells. The ciliary rosettes in the canals may help to transport nutrients to muscles in the mesoglea. The anal pores may eject unwanted small particles, but most unwanted matter is regurgitated via the mouth.[5]
Little is known about how ctenophores get rid of waste products produced by the cells. The ciliary rosettes in the gastrodermis may help to remove wastes from the mesoglea, and may also help to adjust the animal's buoyancy by pumping water into or out of the mesoglea.[5]
Locomotion
The outer surface bears several rows of "combs", which are used for swimming. The rows run from near the mouth to the opposite end, and are usually spaced evenly round the body.[2] The "combs" run across across each row, and each consists of thousands of unusually long cilia, up to 2 millimetres (0.079 in). These normally beat so that the propulsion stroke is away from the mouth, although they can also reverse direction. Hence ctenophores usually swim in the direction in which the mouth is pointing, unlike jellyfish.[5] When trying to escape predators, they can accelerate to six times times their normal speed.[14]
It is uncertain how ctenophores control their buoyancy, but possibly they rely on osmotic pressure to adapt to water of different densities. Their body fluids are normally as concentrated as seawater. If they enter less dense brackish water, the ciliary rosettes in the body cavity may pump this into the mesoglea to increase its bulk and decrease its density, to avoid sinking. Conversely if they move from brackish to to full-strength seawater, the rosettes may pump water out of the mesoglea to reduce its volume and increase its density.[5]
Nervous system and senses
Ctenophores have no brain or central nervous system, but instead have a nerve net (rather like a cobweb) that forms a ring round the mouth and is densest near structures such as the comb rows, pharynx, tentacles (if present) and the sensory complex furthest from the mouth.[5]
The largest single sensory feature is the aboral organ (at the opposite end from the mouth). Its main component is a statocyst, a balance sensor consisting of a statolith, a solid particle supported on four bundles of cilia, called "balancers", that sense its orientation. The statocyst is protected by a transparent dome made of long, immobile cilia. A ctenophore does not automatically try to keep the statolith resting equally on all the balancers. Instead its responses is determined by the animals' "mood", in other words the overall state of the nervous system. For example if a ctenophore with trailing tentacles captures prey, it will often put some comb rows into reverse, spinning the mouth towards the prey.[5]
Symmetry and axes
Since the body of many species is almost radially symmetrical, the main axis is oral to aboral (from the mouth to the oposite end). However since only two of the canals near the statocyst terminate in anal pores, ctenophores have no mirror-symmetery, although many have rotational symmetry, in other words if the animal rotates in a half-circle it looks the same as when it started.[15]
Cydippids
Cydippids, such as the "sea gooseberry" Pleurobranchia, have egg-shaped bodies with the mouth at the narrow end.[5] From opposite sides of the body extends a long, slender tentacle, each housed in a sheath into which it can be withdrawn.[2] Cydippids' bodies may be slightly flattened so that they are wider in the plane of the tentacles.[5]
Each tentacle is fringed with tentilla ("little tentacles") that bear many colloblasts that capture prey by sticking to it. Colloblasts are specialized mushroom-shaped cells in the outer layer of the epidermis, and have three main components: a domed head with vesicles (chambers) that contain adhesive; a stalk that anchors the cell in the lower layer of the epidermis or in the mesoglea; and a spiral thread that coils round the stalk and is attached to the head and to the root of the stalk. The function of the spiral thread is uncertain, but it may absorb stress when prey tries to escape, and thus prevent the collobast from being torn apart. In addition members of the genus Haeckelia , which feed mainly on jellyfish, incorporate their victims' stinging nematocytes into their own tentacles. Euplokamis' tentacles have additonal muscles that enable them to coil round prey.[5][3]
There are eight rows of combs that run from near the mouth to the opposite end, and are spaced evenly round the body.[2] The "combs" beat in a sequence rather like that of a Mexican wave.[16] From each balancer in the statocyst a ciliary groove runs out under the dome and then splits to connect with two adjacent comb rows, and in some species runs all the way along the comb rows. This forms a mechanical system for transmitting the beat rhythm from the balancers to the combs, via water disturbances created by the cilia.[5]
Lobates

The Lobata have a pair of lobes, muscular extensions of the body that project ahead of the mouth. Their small tentacles run in convoluted grooves over the inner surface of the lobes. Between the lobes and surrounding the mouth are four auricles, projections fringed with cilia that produce water currents that wash microscopic prey into the mouth. This combination of structures enables lobates to feed continuously on suspended prey such as plankton.[5]
There are eight comb-rows, two on each lobe and two on each side beteeen the lobes. Members of the genera Leucothea and Ocyropsis also use their large lobes for swimming.[5][5] Unlike cydippids, the movements of lobates' combs are co-ordinated by nerves rather than by via water disturbances created by the cilia, and combs on the same row beat together rather than in Mexican wave style. This may have enabled lobates to grow larger than cydippids and to have shapes that are less egg-like.[16] In addition members of the lobate genera Bathocyroe and Ocyropsis can escape from danger by clapping their lobes, so that the jet of expelled water drives them backwards very quickly.[17]
An unusual species first described in 2000 has been classified as a lobate, although the lobes are "primitive" and the body is medusa-like when floating and disk-like when resting on the sea-bed.[12]
Beroids
The Beroida, also known as Nuda, have no feeding appendages, but their large pharynxes, just inside their large mouths, bear "macrocilia", fused bundles of several thousand large cilia that bite off pieces of prey that is is too large to swallow whole - almost always other ctenophores.[5] Just behind the ring of macrocilia is an internal ridge that runs all round the mouth and "zips" it shut when the animal is not feeding, by forming intracellular connections with the opposite part of the ridge. This tight closure streamlines the front of the animal when it is pursuing prey.[5]
Other body forms
The Ganeshida have a pair of small oral lobes and a pair of tentacles. The body is circular rather than oval in cross-section, and the pharynx extends over the inner surfaces of the lobes.[5]
The Thalassocalycida, only discovered in 1978 and known from only one species,[18] are medusa-like, with bodies that are shortened in the oral-aboral direction, and short comb-rows on the surface furthest from the mouth. They capture prey by movements of the bell and possibly by using two short tentacles.[5]
The Cestoida ("belt animals"), are ribbon-shaped planktonic animals, with the mouth and aboral organ in the middle of opposite edges of the ribbon. There is a pair of comb-rows along each aboral edge, and tentilla on the oral edge. Cestoids can swim by undulating their bodies as well as by means of their comb-rows. The two known species, Cestum veneris ("Venus' girdle") and Velamen parallelum, are the largest ctenophores – up to 1.5 metres (4.9 ft) and 90 centimetres (3.0 ft) long respectively.[5]
The Platyctenida have oval bodies that are flattened in the oral-aboral direction, with a pair of tentilla-bearing tentacles on the aboral surface. They cling to and creep on surfaces by everting the pharynx and using it as a muscular "foot". All but one of the known platyctenid species lack comb-rows.[5]
Reproduction and development

Adults of most species can regenerate tissues that are damaged or removed.[19] However only platyctenids reproduce by cloning, splitting off from the edges of their flat bodies fragments that develop into new individuals.[5]
Almost all species are hermaphrodites, in other words function as both males and females at the same time - except that in two species of the genus Ocryopsis individuals remain of the same single sex all their lives.[5] Fertilization is external fertilization and self-fertilization has occasionally been seen in species of the genus Mnemiopsis,[5] and it is thought that most of the hermaphroditic species are self-fertile.[3]
Development of the fertized eggs is direct, and juveniles of all groups generally resemble miniature cydippid adults. However in the genus Beroe the juveniles, like the adults, lack tentacles and tentacle sheaths. In most species the juveniles gradually develop the body forms of their parents. However in some groups, such as the flat, bottom-dwelling platyctenids, the juveniles behave more like true larvae, as they: live among the plankton and thus occupy a different ecological niche from their parents; and attain the adult form by a more radical metamorphosis,[5] after dropping to the sea-floor.[3]
Juvenile ctenophores appear capable of producing small quantities of eggs and sperm while they are well below adult size, and adults produce eggs and sperm for long as they have sufficient food. If they run short of food, first they stop producing eggs and sperm, and then they shrink in size. When the food suppply improves, they grow back to normal size and then resume reproduction.[3]
Colors and bioluminescence
 |
Most ctenophores that live near the surface are colorless and almost transparent. However some deeper-living species are strongly colored, for example the species known as "Tortugas red", which has been named Agmayeria tortugensis[20] but has not been described in detail.[3] Platyctenids generally live attached to other sea-bottom organisms, and have similar colors to these organisms'.[3] The gut of the deap-sea genus Bathocyroe is red, which hides the bioluminescence of copepods it has swallowed.ref name="Haddock2007ComparativeFeeding" />
Many planktonic ctenophores produce a rainbow effect, which is not caused by bioluminescence but by the scattering of light as the combs move.[3] Most species are also bioluminescent, but the light is usually blue or green and can only been seen in darkness.[3] However some significant groups, including all known platyctenids and the cydippid genus Pleurobrachia, appear incapable of bioluminescence.[21]
When some species, including Bathyctena chuni, Euplokamis stationis and Eurhamphaea vexilligera, are disturbed, they produce secretions that luminesce at much the same wavelengths as their bodies. Juveniles can luminesce more brightly in relation to their body size than adults, whose luminescence is diffused over their bodies. Detailed statistical investigation has not suggested the function of ctenophores' bioluminescence nor produced any correlation between its exact color and any aspect of the animals' environments, such as depth or whether they live in coastal or mid-ocean waters.[22]
Ecology
Distribution
Ctenophores are found in most marine environments: from polar waters to the tropics; near coasts and in mid-ocean; from the surface waters to the ocean depths.[3] However the best-understood are the genera Pleurobrachia, Beroe and Mnemiopsis, as these planktonic coastal forms are easiest to study.[13][17]
Prey and predators
Almost ctenophores are predators – there are no vegetarians and only one genus that is sometimes parasitic.[17] While Beroe preys mainly on other ctenophores, other surface-water species prey on plankton ranging in size from microscopic, including mollusc and fish larvae, to small adult crustaceans such as copepods, amphipods, and even krill. Members of the genus Haeckelia prey on jellysfish and incorporate their prey's nematocysts (stinging cells) into their own tentacles instead of collobasts.[3] They have been compared to spiders in their wide range of techniques from capturing prey - some hang motionless in the water using their tentacles as "webs", some are ambush predators like Salticid jumping spiders, and some dangle a sticky droplet at the end of a fine thread, as Bola spiders do. This variety explains the wide range of body forms in a phylum with rather few species.[17] It also allows some planktonic forms to produce large populations while sharing the same waters, as they prefer different prey.[citation needed] The two-tentacled "cydippid" Lampea feeds exclusively on salps, close relatives of sea-squirts that form large chain-like foating colonies, and juveniles of Lampea attach themselves like parasites to sapls that are too large for thme to swallow.[17]
Invasive species
Although ctenophores are generally hardly noticeable and their influence on an ecosystem is ostensibly very low, they can still do significant damage when they occur in non-native waters. The North Atlantic species Mnemiopsis leidyi was brought by ships' ballast water into the Black Sea and spread rapidly. Mnemiopsis leidy disrupts an ecosystem by preying on large amounts of zooplankton, eggs, and larvae of fish and other invertebrates that would support more desirable species within that ecosystem. Within ten years the anchovy fishing industry around the sea had collapsed, as the newly introduced species fed on the same plankton as the anchovy larvae. The biomass of ctenophora in the Black Sea reached a million tons at the highest point of its development.
Through the similarly sudden appearance in 1997 of another ctenophore, Beroe ovata, which feeds on Mnemiopsis leidyi, the balance was somewhat restored; since then the Black Sea has been occupied by both foreign species. The same scenario with the same species has now begun to be played out in the Caspian Sea, and M. leidyi was also reported from the North Sea in 2006.
The same scenario is now awaited in the Baltic Sea, where Finnish scientists have found that Mnemiopsis gardeni have survived the winter and spread very quickly. A recent expedition found over 600 Mnemiopsis ctenophores per cubic meter in the greater depths of the Baltic Sea.[23]
Mnemiopsis leidyi is a comb jelly native to the Atlantic coastal region and brings in the imbalance in ecosystem by eating huge amount of zooplankton, eggs, and the larvae of fish and invertebrates that would otherwise support populations of more desirable species
Ecology and life history
Habitat
All ctenophora live in the sea, where they live in depths of up to four kilometres. There are no freshwater ctenophores. As plankton they are largely subject to movement of ocean currents, although various species are particular to certain habitats. They can be found in abundance in the tropics and to both poles.
The most well-known species live as plankton in the ocean layers near the surface. However, as they are largely transparent, extremely fragile and rarely grow longer than a few centimetres, they are unknown to most people. On the coast Pleurobrachia species (called sea gooseberries) are encountered most frequently by beachgoers. Bolinopsis, Mnemiopsis and the tentacle-less Beroe can also be found fairly frequently.
About 35 species are not planktonic, but live either on the bottom or attached to other organisms including seaweed, starfish, sponges and other invertebrates.[24] These species are ordered in the taxon of Platyctenida, due to their flattened forms which more closely resemble slugs or flatworms than jellyfish.
The ctenophore Mertensia ovum is one of the most predominant members of plankton in arctic waters.
Community Ecology

Ctenophora are predators which use their tentacles to catch plankton, larvae, worms, crustaceans, cnidaria, other ctenophora, and sometimes small fish. When their tentacles are loaded with food, they can be retracted and wiped off. The food is then carried into the stomach either by mucus or inner cilia. The species of the genus Haeckelia feed almost exclusively on cnidaria, but do not digest their cnidocytes; instead they build them into their own tentacles as 'kleptocnidae'. This 'theft' baffled zoologists for a long time as they falsely assumed ctenophora were also capable of forming cnidocytes. Parasitism has only been observed in a single genus, Lampea, which is parasitic on salps when too small to engulf them entirely.
Among the species that prey on ctenophora are jellyfish, sea turtles, various fish such as chum salmon, mackerels and lumpfish, seabirds and other ctenophora. They are often infested with parasitic crustaceans of the group Amphipoda, and may also have internal parasitic trematodes. Some also carry parasitic dinoflagellates of the genus Oodinium on their comb rows.[25]
Life History

Ctenophora reproduce sexually. Some species of the order Platyctenida are also able to reproduce asexually. Almost all ctenophores are hermaphroditic, or monoecious, possessing both male and female reproductive organs, which lie directly under the 'combs' near the small channels of the mesogloea. In the few cases where it has been carefully examined, these hermaphroditic species seem to be self-fertile, that is, the eggs of an individual can be activated and fertilized by sperm produced by the same animal. Lobate ctenophores in the warm-water genus Ocyropsis are unusual in having separate-sexed individuals. In almost all species, spawning is triggered by changes in outside lighting conditions, and the gametes are discharged into the surrounding water through small openings in the ectoderm, called gonopores, where external fertilisation takes place. Internal fertilization is unusual; the platyctene Tjalfiella tristoma, like some other platyctenes, is viviparous; that is, the young grow in an internal brood chamber until they swim away as a planktonic stage before settling again on the bottom.
Certain species of ctenophores, like Beroe ovata, have a special method of preventing polyspermy. After several sperm pronuclei have entered the egg, the egg pronucleus goes through a process where it migrates around the cell and finally chooses which sperm pronucleus it wants to fuse with, rejecting others because of signals indicating close relationship or lack of fitness.
After the fertilised eggs have divided twice, the ctenophore's later radial body symmetry has already been set. They develop into a free-floating cydippid state, which looks very similar between all ctenophora and sometimes is labeled as a larva, although in many cases this already represents a miniature version of what the creature will grow up to be. Among some groups such as lobates and platyctenids, the cydippid and adult forms do differentiate morphologically, so that the 'larva' label is more appropriate.
Etymology and Taxonomic history
The soft bodies of ctenophores, which have no hard parts whatsoever, makes fossilisation generally very improbable, meaning that the phylogeny of ctenophoran fossils is very sparsely documented. The sole fossil records, of Archaeocydippida hunsrueckiana and Paleoctenophora brasseli, date from the Devonian Period; enough details remained in the fine-grained schist of Hunsrück to make identification possible. It is disputed whether the species Maotianoascus octonarius, known from the Chengjiang Fauna of the lower Cambrian Period, is a member of the ctenophore phylum, while three species, Ctenorhabdotus capulus, Fasciculus vesanus and Xanioascus canadensis, are known from the Cambrian Burgess Shale.
Early classification
Sailors have observed ctenophores since ancient times. However, the first recorded sighting only came in 1671, made by a ship's doctor. The Swedish taxonomist Carl von Linné classified them with other 'primitive' invertebrates such as sea sponges (Porifera) or Cnidaria as 'zoophytes' ("animal plants"), alluding to the passive, "plant-like" character of the creatures. The French zoologist Georges Cuvier supported this classification. Only in the 19th century was ctenophora recognised as a standalone taxon.
Historical phylum
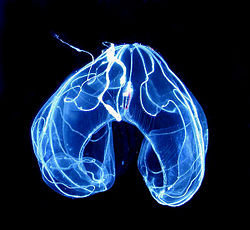
The classification of ctenophora has changed over time. They were originally thought to be related to the superficially similar Cnidaria, in a group called Coelenterata. However, morphological analysis suggests that they may be more closely related to the bilaterally symmetrical Bilateria, in a group called Acrosomata. This is supported by their two opposing tentacles, which break their radial symmetry and making them bilaterally symmetrical (though other structures maintain a rotational or biradial symmetry), by their possession of true muscle tissue, sticky colloblasts in place of cnidocytes, and their 'combs', all of which the Cnidaria lack. Additional evidence is the form of their spermatozoa, which consist of a single, large acrosome and a subacrosomic perforation disc as in the Bilateria, not multiple acrosomic vesicles as in the Cnidarian. Thus before the advent of genetic evidence, there were two principal classifications:
Coelenterata hypothesis
- Eumetazoa
- Bilateria
- Coelenterata
- Cnidaria
- Ctenophora
Acrosomata hypothesis
- Eumetazoa
- Cnidaria
- Acrosomata
- Bilateria
- Ctenophora
In addition it has been suggested that ctenophora have a close relationship with flatworms, due to the similarities between flatworms and the flattened ctenophora of the order Platyctenida, though this resemblance may be superficial.
Current status
In 2008, a phylogenomic study of 150 genes in 21 genera[1] placed ctenophores as the sister group to all other animals included in the analysis, including sponges. The divergence of ctenophores from other animals prior to sponges would indicate that the most recent common ancestor of living animals was more complex than previously believed and that sponges are secondarily simplified (or that ctenophores evolved a nervous system and tissues independently of all other animals). The placement of ctenophores in this position had not been recovered in any previous analyses of smaller datasets. The authors of the study stated that this new finding should be treated provisionally despite its strong support, at least until further data from other animals is available (particularly from more sponges and the placozoan Trichoplax). It is also possible that the finding is an artifact due to the long phylogenetic stem leading to living ctenophores, a possibility that requires more detailed phylogenetic analyses in addition to more data to rule out.
Classification
Currently about 100-150 species are known (many old descriptions are difficult to justify with known species, so it is hard to tell how many constitute unique species)[24], which are traditionally split into the classes of Tentaculata (also known as Tentaculifera) and Nuda (also known as Atentaculata).
- The Tentaculata make up by far the largest number of species; as their name implies, they possess tentacles, although these are sometimes vestigial. They are divided into the following eight orders:
- Cydippida, which includes the sea gooseberry (Pleurobrachia pileus)
- Platyctenida
- Cambojiida
- Ganeshida (probably larval form)
- Cryptolobiferida
- Thalassocalycida
- Lobata
- Cestida, which includes the Venus' girdle (Cestum veneris)
- The Nuda class contains only a single order, Beroida, to which the melon jelly (Beroe gracilis) belongs. As again the name of the taxon implies, they are distinguished by the complete absence of tentacles.
Due to the continued uncertainty over the ordering of ctenophora it is currently unclear whether the above divisions correctly reflect the actual phylogeny of the taxon. Molecular genetic studies indicate that cydipidda is a polyphyletic group, i.e. it does not include all the descendents of their common ancestor, and so the overall classification of the group needs to be revised.
The following diagram shows the putative phylogeny of ctenophora on the basis of morphologic and molecular genetic data (RNA):
Ctenophora
|--Cydippida (Mertensiidae family)
|--
|--Platyctenida
|--
|--Cydippida (Pleurobrachidae family)
|--
| |--Nuda Beroida
| |--Cydippida (Haeckeliidae family)
|
|--
|--Lobata
|--Cestida
|--Thalassocalycida
The above details are however still in doubt. For the time being the phylogeny of ctenophora must be regarded as unsettled.
Bibliography
- Some of this article is based on a translation of the corresponding German-language Wikipedia article, retrieved on 5 April 2006.
- D. T. Anderson, Invertebrate Zoology, 2nd ed, Oxford Univ. Press, 2001, Ch. 3, p. 54, ISBN 0-19-551368-1
- R. S. K. Barnes, P. Calow, P. J. W. Olive, D. W. Golding, J. I. Spicer, The invertebrates – a synthesis, 3rd ed, Blackwell, 2001, ch. 3.4.3, p. 63, ISBN 0-632-04761-5
- R. C. Brusca, G. J. Brusca, Invertebrates, 2nd Ed, Sinauer Associates, 2003, ch. 9, p. 269, ISBN 0-87893-097-3
- J. Moore, An Introduction to the Invertebrates, Cambridge Univ. Press, 2001, ch. 5.4, p. 65, ISBN 0-521-77914-6
- E. E. Ruppert, R. S. Fox, R. P. Barnes, Invertebrate Zoology – A functional evolutionary approach, Brooks/Cole 2004, ch. 8, p. 181, ISBN 0-03-025982-7
- W. Schäfer, Ctenophora, Rippenquallen, in W. Westheide and R. Rieger: Spezielle Zoologie Band 1, Gustav Fischer Verlag, Stuttgart 1996
- Bruno Wenzel, Glastiere des Meeres. Rippenquallen (Acnidaria), 1958, ISBN 3-7403-0189-9
- Mark Shasha, Night of the Moonjellies, 1992, Simon & Schuster, ISBN 0-6717-7565-0
References
- ^ a b Dunn et al. 2008. "Broad phylogenomic sampling improves resolution of the animal tree of life". Nature 06614.
- ^ a b c d e f g h i j k l Hinde, R.T., (1998). "The Cnidaria and Ctenophora". In Anderson, D.T., (ed.). Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 0195513681.
{{cite book}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o Mills, C.E. "Ctenophores - some notes from an expert". Retrieved 2009-02-05.
- ^ Viitasalo, S., Lehtiniemi, M., and Katajisto, T. (2008). "The invasive ctenophore Mnemiopsis leidyi overwinters in high abundances in the subarctic Baltic Sea". Journal of Plankton Research. 30 (12): 1431–1436. doi:10.1093/plankt/fbn088.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 182–195. ISBN 0030259827.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Trumble, W., and Brown, L. (2002), "Ctenophore", Shorter Oxford English Dictionary, Oxford University Press
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 111–124. ISBN 0030259827.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Seipel, K., and Schmid, V. (June 2005). "Evolution of striated muscle: Jellyfish and the origin of triploblasty". Developmental Biology. 282 (1): 14–26. doi:10.1016/j.ydbio.2005.03.032.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 76–97. ISBN 0030259827.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Bergquist, P.R., (1998). "Porifera". In Anderson, D.T., (ed.). Invertebrate Zoology. Oxford University Press. pp. 10–27. ISBN 0195513681.
{{cite book}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Exposito, J-Y., Cluzel, C., Garrone, R., and Lethias, C. "Evolution of collagens". The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology =. 268: 302–316. doi:10.1002/ar.10162.
{{cite journal}}: Cite has empty unknown parameter:|unused_data=(help); Text "issue3" ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Horita, T. (March 2000). "An undescribed lobate ctenophore, Lobatolampea tetragona gen. nov. & spec. nov., representing a new family, from Japan". Zool. Med. Leiden. 73 (30): 457–464. Retrieved 2009-01-03.
- ^ a b Haddock, S.H.D (2004). "A golden age of gelata: past and future research on planktonic ctenophores and cnidarians" (PDF). Hydrobiologia. 530/531: 549–556. Retrieved 2009-02-03.
- ^ Kreps, T.A., Purcell, J.E., and Heidelberg, K.B. Marine Biology. 128 (3): 441–446. doi:10.1007/s002270050110.
{{cite journal}}: Missing or empty|title=(help); Text "Escape of the ctenophore Mnemiopsis leidyi from the scyphomedusa predator Chrysaora quinquecirrha" ignored (help); Text "June 1997" ignored (help)CS1 maint: multiple names: authors list (link) - ^ Martindale, M.Q., and Henry, J.Q. (October 1999). "Intracellular Fate Mapping in a Basal Metazoan, the Ctenophore Mnemiopsis leidyi, Reveals the Origins of Mesoderm and the Existence of Indeterminate Cell Lineages". Developmental Biology. 214 (2): 243–257. doi:10.1006/dbio.1999.9427.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Craig, C.L., and and Okubo, A. (April 1990). "Physical constraints on the evolution of ctenophore size and shape". Evolutionary Ecology. 4 (2): 115–129. doi:10.1007/BF02270909.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Haddock, S.H.D. (2007). "Comparative feeding behavior of planktonic ctenophores". Integrative and Comparative Biology. 47 (6): 847–853. doi:10.1093/icb/icm088.
- ^ Gibbons, M. J., Richardson, A. J., Angel, M. V., Buecher, E., Esnal, G., Fernandez Alamo, M. A., Gibson, R., Itoh, H., Pugh, P., Boettger-Schnack, R. and Thuesen, E. (March 2005). "What determines the likelihood of species discovery in marine holozooplankton: is size, range or depth important?" (PDF). Oikos. 109: 567–576. doi:10.1111/j.0030-1299.2005.13754.x. Retrieved 2009-01-03.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Martindale, M.Q. (December 1986). "The ontogeny and maintenance of adult symmetry properties in the ctenophore, Mnemiopsis mccradyi". Developmental Biology. 118 (2): 556–576. PMID 2878844.
- ^ Mills, C.E. "Phylum Ctenophora: list of all valid scientific names". Retrieved 2009-02-10.
- ^ Haddock, S.H.D. (1995). "Not All Ctenophores Are Bioluminescent: Pleurobrachia" (PDF). Biological Bulletin. 189: 356–362. Retrieved 2009-02-10.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Haddock, S.H.D., and Case, J.F. (April 1999). "Bioluminescence spectra of shallow and deep-sea gelatinous zooplankton: ctenophores, medusae and siphonophores" (PDF). Marine Biology. 133: 571–582. doi:10.1007/s002270050497. Retrieved 2009-02-10.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Internytt, 2007-08-27
- ^ a b Mills, C.E. (March 1998 to present). "Phylum Ctenophora: list of all valid species names". Retrieved 2008-09-02.
{{cite web}}: Check date values in:|date=(help) - ^ Mills, C.E. (1991). "Ectoparasitism by a dinoflagellate (Dinoflagellata: Oodinidae) on 5 ctenophores (Ctenophora) and a hydromedusa (Cnidaria)". Diseases of Aquatic Organisms. 10: 211–216.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
http://www.hoxfulmonsters.com/2009/01/meet-the-most-primitive-animal-group-ctenophores/
External links
- C.E. Mills - General introduction to ctenophores
- Ctenophores from the São Sebastião Channel, Brazil
- Video of ctenophores at the National Zoo in Washington DC
- Tree Of Animal Life Has Branches Rearranged, By Evolutionary Biologists
- Australian Ctenophora Fact Sheet
- The Jelly Connection – striking images, including a Beroe specimen attacking another ctenophore



