Phagocyte: Difference between revisions
Brianboulton (talk | contribs) |
→Avoiding engulfment: a biofilm is made of bacteria; a coating of polysaccharides on a bacterium's surface is totally different, and much smaller |
||
| Line 176: | Line 176: | ||
===Avoiding engulfment=== |
===Avoiding engulfment=== |
||
Bacteria usually have a component in their cell wall that allows them to resist engulfment by a phagocyte.<ref name=chicken>{{cite web|url=http://textbookofbacteriology.net/antiphago.html|title=MECHANISMS OF BACTERIAL PATHOGENICITY: Bacterial Defense Against Phagocytes|accessdate=2008-12-10|last=Todar|first=Kenneth|publisher=2008}}</ref> An example of this is the K5 capsule and the O75 [[O antigen]] found on the surface of ''[[Escherichia coli]]'' used to prevent phagocytosis.<ref>{{cite journal|title=Loss of resistance to ingestion and phagocytic killing by O(-) and K(-) mutants of a uropathogenic Escherichia coli O75:K5 strain.|journal=Infection and immunity.|date=1999|first=SM|last=Burns|coauthors=SI Hull|volume=67|issue=8|pages=3757–62|pmid=10417134|url=http://www.ncbi.nlm.nih.gov/pubmed/10417134|accessdate=2008-12-10}}</ref> Bacteria can also produce |
Bacteria usually have a component in their cell wall that allows them to resist engulfment by a phagocyte.<ref name=chicken>{{cite web|url=http://textbookofbacteriology.net/antiphago.html|title=MECHANISMS OF BACTERIAL PATHOGENICITY: Bacterial Defense Against Phagocytes|accessdate=2008-12-10|last=Todar|first=Kenneth|publisher=2008}}</ref> An example of this is the K5 capsule and the O75 [[O antigen]] found on the surface of ''[[Escherichia coli]]'' used to prevent phagocytosis.<ref>{{cite journal|title=Loss of resistance to ingestion and phagocytic killing by O(-) and K(-) mutants of a uropathogenic Escherichia coli O75:K5 strain.|journal=Infection and immunity.|date=1999|first=SM|last=Burns|coauthors=SI Hull|volume=67|issue=8|pages=3757–62|pmid=10417134|url=http://www.ncbi.nlm.nih.gov/pubmed/10417134|accessdate=2008-12-10}}</ref> Bacteria can also produce a mix of various sugar polymers called [[exopolysaccharide]] on their surface that inhibits phagocytosis. This is seen in ''[[Staphylococcus epidermidis]]''—to avoid engulfment, it produces a biofilm composed of poly-N-acetylglucosamine.<ref>{{cite journal|title=A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence.|journal=The journal of biological chemistry|date=2004-12-24|first=C|last=Vuong|coauthors=S Kocianova, JM Voyich, Y Yao, ER Fischer, FR Deleo, M Otto|volume=279|issue=52|pages=54881-6|pmid=15501828|url=http://www.ncbi.nlm.nih.gov/pubmed/15501828|accessdate=2008-12-10}}</ref> Some bacteria use a [[polysaccharide]] capsule as a shield against phagocytic engulfment. This is done by ''[[Streptococcus pneumoniae]]''—there are several types of capsules that are used, all with different levels of protections.<ref>{{cite journal|title=Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B.|journal=Infection and immunity.|date=2008-12-1|first=M|last=Melin|coauthors=H Jarva, L Siira, S Meri, H Käyhty, M Väkeväinen|pmid=19047408|url=http://www.ncbi.nlm.nih.gov/pubmed/19047408?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum|accessdate=2008-12-10}}</ref> ''[[Group A streptococci]]'' use surface proteins such as [[M protein]] and [[fimbriae|fimbrial proteins]] to block engulfment. Proteins can also be used to hinder antibody related ingestion. ''[[Staphylococcus aureus]]'' does this by using [[Protein A]] (it attaches to the Fc receptor to decrease the effectiveness of IgG antibodies).<ref name="pmid16322743">{{cite journal| author = Foster TJ| title = Immune evasion by staphylococci| journal = Nat. Rev. Microbiol.| volume = 3| issue = 12| pages = 948–58| year = 2005| month = December| pmid = 16322743| doi = 10.1038/nrmicro1289| url = http://dx.doi.org/10.1038/nrmicro1289| accessdate = 2008-12-13}}</ref> |
||
===Survival inside the phagocyte=== |
===Survival inside the phagocyte=== |
||
Revision as of 03:23, 26 February 2009
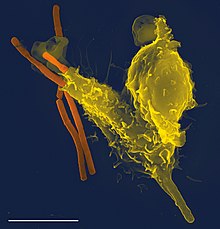
Phagocytes are living cells that move within the body and ingest other cells which are dead or dying, pathogenic, infectious and other foreign particles.[1] They are the basis of defense in the innate immune system and play a central role in the adaptive immune system.[1] Phagocytes are found in the blood, bone marrow and other tissues of vertebrates,[1] and in the hemolymph of invertebrates.[2] In vertebrates they originate in the bone marrow and derive from a group of stem cells called myeloid progenitor cells. In invertebrates they are formed in the lymph glands.[2] Their name is from the Greek phagein, 'to eat or devour', and kutos, 'hollow vessel'.[3]
Phagocytes of humans and other higher animals are divided into "professional" and "non-professional" groups based on the efficiency with which they phagocytose.[4] The professional phagocytes include neutrophils, monocytes, tissue macrophages, dendritic cells, and mast cells.[5] The main distinguishing factor between professional and non-professional phagocytes is that professional phagocytes have receptors on their surfaces that aid in phagocytosis.[6]
During an infection molecules are produced that attract phagocytes to the site of infection. These molecular signals may come from bacteria, complement factors, or tissue macrophages already at the site of infection. The phagocytes follow the signal to the site of infection through a process called chemotaxis. When the phagocyte comes in contact with dying cells or invading agents, they bind to the receptors on the phagocyte's surface and are engulfed. Many species of bacteria and other pathogens have evolved methods to counter attacks by phagocytes.[1]
After phagocytosis, macrophages and dendritic cells may participate in antigen presentation, in which the phagocyte moves molecules from the pathogen or its by-products to the surface of the phagocyte. On re-entering the circulation system of the host, the phagocyte "presents" these antigens to other cells of the immune system, and the immune response is amplified.[7] Dendritic cells also have the ability to promote immunological tolerance;[8] this prevents the body from attacking itself.[9]
Phagocytes were first discovered in 1882 by Ilya Ilyich Mechnikov while he was studying the larvae of starfishes.[10] For his findings Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine along with Paul Ehrlich.[11] Phagocytes occur in many species; some amoebae behave like macrophages and also have the ability to distinguish between self and non-self when feeding. This suggests that phagocytes appeared early in the evolution of life.[12]
History

During the late nineteenth and early twentieth centuries a debate developed between the supporters of the cellular and humoral theories of immunity. Ilya Ilyich Mechnikov was a supporter of the cellular theory;[13] in 1882, he studied motile cells in the larvae of starfishes that he believed were important to their immune defenses. To test his idea he inserted small thorns from a tangerine tree into the larvae. He noticed that the motile cells surrounded the thorns. Mechnikov knew that in animals with a vascular system, leukocytes migrate from the blood stream during an infection. He deduced that these leukocytes had the ability to migrate from the blood to engulf and digest bacteria. Mechnikov traveled to Vienna and shared his ideas with Carl Friedrich Claus (1835–99),[14] who suggested the name ‘‘phagocyte’’.[15] To advance his hypothesis, Mechnikov studied a fresh-water crustacean called Daphnia. He discovered that fungal spores that attacked the crustacean were destroyed by phagocytes. He later studied the bacterium Bacillus anthracis and found that this organism could also be destroyed by phagocytes.[10] Mechnikov proposed that phagocytes were a primary defense against invading organisms and he and Paul Ehrlich were jointly awarded the 1908 Nobel Prize in Physiology or Medicine for their work on phagocytes and phagocytosis.[11] Although the importance of their discoveries slowly gained acceptance during the early twentieth century, the intricate relationships between phagocytes and virtually all other components of the immune system were not known until much later.[16] Phagocytes of humans and other higher animals are divided into "professional" and "non-professional" groups based on the efficiency with which they phagocytose.[4] The principal professional phagocytes are now known to be the neutrophils, monocytes, tissue macrophages, dendritic cells and possibly mast cells.[5]
Professional phagocytes
Monocytes
Most mature monocytes have large, smooth, bi-lobed nuclei and abundant cytoplasm that contains granules. Monocytes ingest foreign or dangerous substances and present antigens to other cells of the immune system. Monocytes form two groups: a circulating group and a marginal group (approximately 70% are in the marginal group). Most monocytes leave the blood stream to travel to tissues and organs, and in doing so transform into macrophages.[17] Monocytes also serve as precursors to dendritic cells.[18]
Macrophages
Macrophages derive from monocytes, granulocyte-monocyte precursors, or the division of pre-existing macrophages.[19] This type of phagocyte does not have granules but contains many lysosomes. Macrophages are found throughout the body in almost all tissues and organs (e.g. microglia cells in the brain and alveolar macrophages in the lungs). A macrophage's location can also determine its size and appearance. Macrophages have many functions: they can ingest cell debris and foreign or harmful cells and antibodies (they are frequently seen with cytoplasmic projections that are used for engulfment), they are involved in antigen presentation, and they can even store iron.[20] Macrophages also participate in inflammation through the production of IL-6, TNF-alpha, and IL-1.[21] Macrophages are usually only found in tissue and are rarely seen in blood circulation. Most have a lifespan of 3–6 weeks.[20]
Macrophages can be activated to perform functions that cannot be performed by a resting monocyte.[21] T helper cells (also known as effector T cells or Th cells), a sub-group of lymphocytes (a type of white blood cell), are responsible for the activation of macrophages. Th1 cells activate macrophages by signaling with IFN-gamma and displaying the protein CD40 ligand.[22] Other signals include TNF-alpha and lipopolysaccharides from bacteria.[21] The signals then allow the macrophage to effectively kill the microbes that were residing in their phagosomes. Th1 cells can recruit other phagocytes in several ways. They secrete cytokines that act on the bone marrow to stimulate the production of monocytes and neutrophils and they secrete some of the cytokines and chemokines that are responsible for the migration of monocytes and neutrophils out of the blood stream.[22] Th1 cells come from the differentiation of CD4 T cells once they have responded to antigen in the secondary lymphoid tissues.[21] Macrophages' NADPH oxidase (an enzyme that plays a role in respiratory bursts) activity increases after activation as well.[23] Activated macrophages also play a more potent role in tumor destruction after activation by producing TNF-alpha, IFN-gamma, nitric oxide, reactive oxygen compounds, cationic proteins, and hydrolytic enzymes.[21]
Neutrophils
Neutrophils participate in phagocytosis of antibody and complement coated antigens. They can also ingest damaged cells or cell parts. Neutrophils are smaller than monocytes, and have a segmented nucleus with several sections; each section is connected by chromatin filaments—neutrophils can have 2–5 segments. Neutrophils do not normally exit the bone marrow until their nucleus has been segmented; but if there is a high need for neutrophils or if there are irregularities in the bone marrow, neutrophil precursors called myelocytes and promyelocytes are released. Neutrophils are also separated between circulating and marginal groups (about 50% of neutrophils are marginated).[24]
The intra-cellular granules of the human neutrophil have long been recognized for their proteolytic and bactericidal properties.[25] Neutrophils can also secrete products that stimulate monocytes and macrophages. Neutrophil secretions increase phagocytosis and the formation of reactive oxygen compounds involved in intracellular killing.[26] Heparin-binding protein and human neutrophil peptides 1–3 have been found to mediate the response to neutrophil secretions. Secretions from the primary granules of neutrophils stimulate the phagocytosis of IgG-coated bacteria.[27]
Dendritic cells

Dendritic cells are specialized antigen-presenting cells that have long outgrowths called dendrites.[28] These cells derive from the bone marrow and are present in small quantities in tissues that are in contact with the external environment, mainly the skin (where there is a specialized dendritic cell type called Langerhans cells) and the inner lining of the nose, lungs, stomach and intestines. They are also found in an immature state in the blood. Once activated, they migrate to the lymphoid tissues where they interact with T cells and B cells to initiate and shape the adaptive immune response.[29] After monocytes have turned into immature dendritic cells, the immature dendritic cells circulate throughout the body. The dendrites help to engulf microbes and other antigen sources in peripheral tissues.[30][31] Once antigens have been engulfed, they are converted into proteolytic peptides and are attached to MHC class I or II molecules.
Following the conversion of antigens into proteolytic peptides, dendritic cells travel to secondary lymphoid organs and mature so that they can present the antigens to T lymphocytes.[31] Mature dendritic cells can produce other products that stimulate T lymphocytes and help orchestrate the immune response. How effective the immune response controlled by dendritic cells is, depends on their maturity. This can be increased through signals from captured microbes and antigens and other factors in the immune system. Dendritic cells also activate both helper T cells and killer T cells. The activated helper T cells also interact with macrophages and B cells to activate them. In addition, dendritic cells can influence the type of immune response; when they travel to the lymphoid areas where T cells are held they select the specific T cells which differentiate into killer T cells and helper T cells.[30]
Mast cells
Mast cells have been found to be involved in both the innate and adaptive immune systems. This stems from evidence such as the mast cells possession of toll-like receptors and the ability of mast cells to interact with dendritic cells, B cells, and T cells to help mediate adaptive immune functions. Mast cells have been shown to express working MHC class II molecules and can participate in antigen presentation. However the mast cell's role in antigen presentation is not very well understood.[32] Mast cells have been shown to phagocytose, kill, and process antigens from gram-negative bacteria,[33] such as Salmonella, and that a mast cell's ability to process antigens is linked to the fimbrial proteins on the surface of bacteria.[34] In addition to these functions mast cells produce cytokines that induce an inflammatory response.[35] This is a vital part of the destruction of microbes because they attract more phagocytes to the site of infection.[33]
In at some primitive vertebrates, B cells can serve as professional phagocytes.[36]
| Location | Variety of phenotypes |
|---|---|
| Bone marrow | macrophages, monocytes, sinusoidal cells, lining cells |
| Bone tissue | osteoclasts |
| Gut and intestinal Peyer's patches | macrophages |
| Connective tissue | histiocytes, macrophages, monocytes |
| Liver | Kupffer cells, monocytes |
| Lung | self-replicating macrophages, monocytes, mast cells |
| Lymphoid tissue | free and fixed macrophages and monocytes |
| Nervous tissue | microglial cells (CD4+) |
| Spleen | free and fixed macrophages, monocytes, sinusoidal cells |
| Thymus | free and fixed macrophages and monocytes |
| Skin | resident Langerhans cells, dendritic cells, conventional macrophages, mast cells |
Non-professional phagocytes
| Variety of phenotypes |
|---|
| Lymphocytes |
| NK and LGL cells (Large Granular Lymphocytes) |
| Epithelial cells |
| Endothelial cells |
| Fibroblasts |
| Erythrocytes |
Apoptotic cells and many organisms are phagocytosed by cells other than macrophages and neutrophils.[38] These non-myeloid cells, which include epithelial, endothelial, and mesenchymal cells, are called non-professional phagocytes, emphasizing that, in contrast to professional phagocytes, phagocytosis is not their principal function.[39]
The major difference between professional and non-professional phagocytes is the possession of receptors for specific opsonins (e.g. IgG Fc and complement receptors), by professional phagocytes.[6] Fibroblasts, for example, only make ineffective attempts to ingest foreign particles.[40]
Phagocytosis

Phagocytosis is the process of taking in foreign material.[41] It is generally defined as the internalization of particles with a diameter of at least 0.5μ, such as bacteria, parasites, dead host cells and cellular and foreign debris.[42] It is one of the endocytic processes,[43] but endocytosis is a fundamentally distinct process.[42] Two distinct mechanisms may lead to the transfer of microorganisms to the cytoplasm. In conventional, or zipper-type, phagocytosis, ingestion occurs by sequential engagement of a phagocyte's membrane with the particle surface, and pseudopod advance proceeds no further than receptor-ligand interaction permits. In macropinocytosis, or trigger-type phagocytosis, in contrast, the host cell forms large surface ruffles or pseudopods in the vicinity of a bound microorganism.[39] Phagocytosis occurs after the bacterium is bound to one of the receptors. In this process the phagocyte stretches its pseudopods around the bacterium and engulfs it. The bacterium is then trapped in a phagosome. The phagosome then combines with either a lysosome or a granule (from a neutrophil). The contents of the granule or lysosome are then released into the phagosome—the combination of a phagosome and a lysosome (or granule) produces a phagolysosome.[1]
Initiation of phagocytosis
A phagocyte has receptors on its surface that are used to bind infectious agents to itself.[1] These receptors increase the ability of a phagocyte to ingest foreign material.[43] These receptors include Fc receptors, complement receptors, scavenger receptors, and toll-like receptors. Fc receptors increase the phagoctyosis of bacteria that have been coated with IgG antibodies. When bacteria coated with IgG antibodies are bound to the Fc receptors, this increases the metabolic activity of phagocytes used in intracellular killing. Complement receptors bind bacteria coated with complement C3b. Binding to the complement receptors increases phagocytosis and intracellular killing. Scavenger receptors bind to a large range of molecules on the surface of bacterial cells, and increase the phagocytosis of bacteria. Toll-like receptors bind to more specific molecules. Binding to toll-like receptors increases phagocytosis and causes the phagocyte to release a group of cytokines related to inflammation.[1] Toll-like receptors are present in vertebrates and invertebrates.[44]
Migration
Initial signaling
When infection occurs, an SOS-like signal is given off to attract monocytes (macrophage[17] and dendritic cell precursors[18]) and neutrophils. Chemical signals may include N-formyl-methionine peptides that originate in invading bacteria, clotting system peptides, complement products, and cytokines that have been given off by macrophages located in the tissue near the infection site.[1] Another group of chemical attractants are chemokines (a type of cytokine) that are released by phagocytes near the infection. Like the other attractants, chemokines serve as recruiting agent for neutrophils and monocytes. For example, interleukin-8 attracts neutrophils from the blood stream into surrounding tissues, and macrophage chemoattractant protein-1 causes monocytes to leave the blood stream and enter tissues near the infection where the monocytes then develop into tissue macrophages.[45]
Endothelial and epithelial migration
Signaling promotes the phagocytes to attach to cell adhesion molecules. Selectins are the first group of endothelial adhesion molecules. Selectins—cytokines from macrophages are responsible for the release of granules found in endothelial cells that contain P-selectins— are found on the membrane of the endothelial cell, and bond with certain carbohydrate groups, like the oligosaccharides on the surface of the monocytes and neutrophils. Intracellular adhesion molecules (or ICAMs) are responsible for producing a tighter attachment to the phagocyte. These molecules form bonds with the integral proteins on the surface of the circulating monocytes and neutrophils. ICAM-1 promotes strong endothelial and phagocytic bonds on the surface of irritated endothelial cells. Chemokines also help to create a better connection by changing the shape of molecules such as leukocyte functional antigen-1 (LFA-1) found on traveling monocytes and neutrophils. While ICAM-1 binds to LFA-1 on both neutrophils and monocytes (after exposure to the macrophage cytokine TNF-a), ICAM-2 is used to help only monocytes get into the infected tissue.[45] Other signals from the infection site called vasodilators enable the phagocytes to cross through the spaces of endothelial cells by loosening the junctions connecting them (a process called diapedesis). Once the phagocytes are in the tissue in which the infection is occurring, chemotaxis allows the phagocytes to find the exact area. SOS signals may also enhance a phagocyte’s ability to ingest and kill organisms through the respective processes of phagocytosis and intracellular killing.[1]
Neutrophils also travel across epithelial-lined organs to sites of infection. This involves a series of interactions that have not yet been fully studied. Several protein interactions that have been identified are those between leukocyte CD11b (and CD18) with fucosylated glycoproteins that have been expressed by signaling. Following this reaction is a binding of the leukocyte proteins and desmosomal-associated JAM-C. Two other binding proteins have also been studied: junctional adhesion molecule-like protein (from the neutrophil) and epithelial coxsackie and adenovirus receptor.[46] Although neutrophil migration across epithelial-lined organs is an important component of fighting infection, the migration itself can result in disease-like symptoms.[47]
Intracellular killing
Oxygen-dependent intracellular killing
The killing of microbes is a critical physiological function of phagocytes.[48] When a phagocyte ingests bacteria (or any material), its oxygen consumption increases. The increase in oxygen consumption is called a respiratory burst. A respiratory burst results in the production of anti-microbial reactive oxygen-containing molecules.[49] Killing invading microbes by using the reactive oxygen-containing molecules is referred to as oxygen-dependent intracellular killing. The oxygen compounds are toxic to both the invader and the cell itself, so the phagocyte uses a series of detoxification reactions to protect itself by breaking down the substances. There are two types of oxygen-dependent intracellular killing methods. The first type is oxygen-dependent myeloperoxidase-independent intracellular killing. When glucose is used during phagocytosis, it is converted into NADPH. Then NADPH oxidase is activated, this enzyme’s role is to oxidize NADPH. The oxidation of NADPH creates superoxide anion.[1] Superoxide anion is an important microbicidal substance in phagocytes.[50] The superoxide anion is then converted to hydrogen peroxide and singlet oxygen with the help of the enzyme superoxide dismutase. In addition to these compounds, superoxide anion reacts with hydrogen peroxide to produce hydroxyl radicals. All of these products are used to kill the invading microbe.[1] The next type, oxygen-dependent myeloperoxidase-dependent intracellular killing, occurs in neutrophils and monocytes because it involves the use of myeloperoxidase from granules.[51] When granules fuse with a phagosome, myeloperoxidase is released into the phagolysosome—this enzyme uses hydrogen peroxide and halide ions (primarily chloride ions) to create hypochlorite. Hypochlorite is an extremely toxic substance that can be broken down by itself into singlet oxygen. Both the hypochlorite and the singlet oxygen are used to kill microbes in the phagolysosome.[1]
Oxygen-independent intracellular killing
Phagocytes can also kill microbes by oxygen-independent methods, but these are not as effective as the oxygen-dependent methods. There are four main types of oxygen-independent methods. The first type uses cationic proteins; when the phagosome becomes a phagolysosome these proteins are released and used to damage the bacterium's membrane. The second type uses lysozymes; these enzymes are used to break down the bacterial cell wall. The third type uses of lactoferrins; they are used to take away iron from the bacterium. The fourth type uses proteolytic and hydrolytic enzymes; these enzymes are used to digest the proteins of killed bacteria.[1]
Extracellular killing
In macrophages, IFN-gamma stimulates the production of nitric oxide by increasing the use of inducible nitric oxide synthase (iNOS). TNF-alpha is also used in this process to promote anti-microbial iNOS methods.[52] Nitric oxide is then released from the macrophage; and, because of its toxicity, kills invading microbes near the macrophage.[1]
Antigen presentation
There are two 'professional' antigen-presenting cells: macrophages and dendritic cells.[53] After phagocytosis, these cells derive antigens from either the pathogen itself or from its products. Protein antigens are turned into peptides inside of the dendritic cells and macrophages; the peptides are then carried to the surface by linking to major histocompatibility complex (MHC) glycoproteins. There are two different classes of MHC molecules that carry peptides originating from different places inside the cell: MHC class I and MHC class II. MHC class I molecules carry peptides from the cytosol to the surface of the cell where CD8 T cells recognize them. MHC class II molecules transport peptides from vesicles to the surface of the cell where they are recognized by CD4 T cells. MHC molecules are both polygenic (the cell possesses several genes that code for each class of MHC molecules, with no allelic exclusion) and polymorphic (the genes have many variations capable of producing different molecules), enabling them to bind and carry a great range of peptides to the surface of a cell for presentation to T cells.[7]
Immunological tolerance
Dendritic cells also serve the function of promoting immunological tolerance.[8] Immunological tolerance is important because it keeps the body from attacking itself. The first type of immunological tolerance is central tolerance: When T-cells first depart from the thymus, dendritic cells destroy the T-cells that carry antigens that would cause the immune system to attack itself. The second type of immunological tolerance is peripheral tolerance. Some T-cells that posses antigens that would cause them to attack self slip through the first process of tolerance, some T-cells develop self-attacking antigens later in life, and some self-attacking antigens are not found in the thymus; because of this dendritic cells again restrain their activity. Dendritic cells can do this by destroying them or by recruiting the help of regulatory T-cells to inactivate the harmful T-cells' activities.[9] When immunological tolerance fails, an autoimmune diseases can follow.[54] On the other hand, too much tolerance allows some infections, like HIV, to go unnoticed.[9]
Role in apoptosis
Dying cells that undergo the final stages of apoptosis display phagocytotic molecules, such as phosphatidylserine, on their cell surface.[55] Phosphatidylserine is normally found on the cytosolic surface of the plasma membrane, but is redistributed during apoptosis to the extracellular surface by a hypothetical protein known as scramblase.[56] These molecules mark the cell for phagocytosis by cells possessing the appropriate receptors, such as macrophages.[57] The removal of dying cells by phagocytes occurs in an orderly manner without eliciting an inflammatory response and is an important function of phagocytes.[58]
Bacterial evasion and resistance
A pathogen is only successful in infecting an organism if it can get past its defenses. Bacteria have developed many different methods to resist attacks by phagocytes.[59]
Avoiding contact
There are several ways bacteria avoid contact with phagocytes. First, they can grow in sites that phagocytes are not capable of traveling to (such as the urinary bladder and the surface of unbroken skin). Second, bacteria can suppress the inflammatory response; without this phagocytes cannot respond effectively. Third, bacteria may inhibit phagocytes' traveling to the site of infection. Bacteria do this by interfering with chemotaxis. Some strains of Mycobacterium tuberculosis hinder leukocyte chemotaxis, and bacteria in the genus Clostridium produce a toxin that inhibits neutrophil migration.[59] Fourth, some bacteria can avoid contact with phagocytes by tricking the immune system into thinking that the bacteria are 'self'. This is demonstrated by Treponema pallidum, the bacterium that causes syphilis. This bacterium coats its surface with fibronectin.[60]
Avoiding engulfment
Bacteria usually have a component in their cell wall that allows them to resist engulfment by a phagocyte.[59] An example of this is the K5 capsule and the O75 O antigen found on the surface of Escherichia coli used to prevent phagocytosis.[61] Bacteria can also produce a mix of various sugar polymers called exopolysaccharide on their surface that inhibits phagocytosis. This is seen in Staphylococcus epidermidis—to avoid engulfment, it produces a biofilm composed of poly-N-acetylglucosamine.[62] Some bacteria use a polysaccharide capsule as a shield against phagocytic engulfment. This is done by Streptococcus pneumoniae—there are several types of capsules that are used, all with different levels of protections.[63] Group A streptococci use surface proteins such as M protein and fimbrial proteins to block engulfment. Proteins can also be used to hinder antibody related ingestion. Staphylococcus aureus does this by using Protein A (it attaches to the Fc receptor to decrease the effectiveness of IgG antibodies).[64]
Survival inside the phagocyte
Bacteria have developed ways to survive inside phagocytes where they are protected from harmful drugs and extracellular bactericidal compounds.[65] However, these bacteria must first get inside the phagocyte and they do this by expressing a protein called invasions (e.g. Salmonella and Legionella). Legionella pneumophila enters phagocytes by coating its surface with the complement factor C3b. There are many methods of survival and stopping the fusion of a phagosome and lysosome into a phagolysosome is one.[59] Legionella pneumophila does this by using a secretion system. These secretions cause the phagosome to fuse with vesicles other than the ones that contain bactericidal compounds. These bacteria also inhibit the trafficking of vesicles and changes the phagosome that they are in.[66] Some bacteria are capable of living inside of the phagolysosome as another means of survival. Staphylococcus aureus does this by producing the enzymes catalase and superoxide dismutase. These enzymes break down bactericidal products (e.g. hydrogen peroxide).[67] Bacteria may also escape from the phagosome before the formation of the phagolysosome as another method of survival. Listeria monocytogenes does this by using a pore forming enzyme called listeriolysin O, and two variants of the bacterial enzyme phospholipase C.[68]
Killing
Bacteria have also developed ways of killing phagocytes.[64] Some of the ways bacteria kill phagocytes before being engulfed include: cytolysins (that form pores in the phagocyte's cell membranes), using streptolysins (this causes a neutrophil’s granules to rupture releasing toxic substances), using leukocidin[69][70] (this also results in the release of the contents in a neutrophil’s granules), and using exotoxins (these toxins can reduce the supply of a phagocyte's ATP, which is needed for phagocytosis). After a bacterium is ingested it may kill the phagocyte by releasing toxins that travel through the phagosome or phagolysosome membrane to target other parts of the cell.[59]
Defects of phagocyte cell function
Several conditions can impair the normal functioning of phagocytes.[71] These are often hereditary but some occur as a result of diseases.[72][73]
- Chemotaxis: These defects occur in rare congenital abnormalities and acquired diseases such as leukemia, myelodysplasia[74] and myeloproliferative syndromes. Phagocyte migration is impaired and causes "lazy leucocyte syndrome".[75]
- Phagocytosis defects are usually caused by a lack of opsonization[76] that results from hypogammaglobulinemia which in turn may be hereditary or acquired.
- Killing defects are seen in the rare chronic granulomatous disease which is an X-linked or autosomal primary immunodeficiency disorder characterized by recurrent infections.[77] In this disease there is an abnormality affecting different elements of the respiratory burst oxidase mechanism. The people affected have recurrent bacterial infections. Other rare congenital abnormalities such as Chediak-Higashi Syndrome are also associated with defective killing of ingested microbes.[78]
Host damage by phagocytes
Although phagocytes play a central role in the inflammatory process and immune response, when engaged in "frustrated phagocytosis" they can cause damage to healthy cells and tissues.[79]
Acute lung injury
Neutrophils are a main component of many acute lung injury (ALI) cases; experiments have shown that a reduction in the number of neutrophils lessens the effects of ALI.[80] The steps to lung damage by neutrophils start with the neutrophil migration through pulmonary microvasculature (this includes the adhesion process). Then neutrophils are activated and begin to fight microbes (with reactive oxygen compounds and proteolytic enzymes).[81] When neutrophils respond to infection they phagocytose the invader and then release granule contents into the phagosome. However, sometimes the granule contents are released outside the cell (this occurs when the release of these substances is unregulated). The microbicidal substances that were released now damage surrounding host tissue. Other compounds (elastase for example) change the pulmonary cells by combining to surface receptors and through signal transduction. These changes may have positive or negative results.[82]
Renal failure
When neutrophils release their granule contents in the kidneys, glomerular cells can be affected in negative ways: changes in the filtration rate and changes in shape. The contents of the granule (reactive oxygen compounds and protease) also degrade the extra-cellular matrix of host cells and can cause damage that cannot be fixed. In addition, phospholipase products (leukotrienes) intensify the damage. Neutrophils also release substances that promote chemotaxis of more neutrophils to the site of infection. When adhesion molecules are used for migration this also damages glomerular cells. The injury done to the glomerular cells can cause renal failure.[83]
Septic shock
TNF-alpha is an important chemical that is released by macrophages: it causes the blood in small vessels to clot (this prevents an infection from spreading). However if an infection spreads to the blood, this helpful chemical can then produce negative results. If the infection has spread to the blood stream TNF-alpha will be released in vital organs (the liver, for example) and can cause vasodilation along with a decrease in plasma volume; this in turn will be followed by shock. Also during septic shock, because TNF-alpha causes clotting, small vessels will be blocked off and many vital organs may fail. Septic shock may lead to death.[45]
Evolutionary origins
Amoebae are free-living phagocytic cells; Dictyostelium discoideum, for example, lives in the soil and feeds on bacteria. This single-celled animal engulfs bacteria by phagocytosis mainly through Toll-like receptors. It is a social amoeba which aggregates when starved to form a migrating slug. This multicellular organism eventually produces a fruiting body with spores that are resistant to environmental dangers. Before the formation of fruiting bodies, the cells can migrate as slug-like organisms for several days. During this time, exposure to toxins or bacterial pathogens have the potential to compromise survival of the amoebae by limiting spore production. Some of the amoebae engulf bacteria and absorb toxins while circulating within the slug and these amoebae eventually die. They are genetically identical to the other amoebae in the slug and their sacrificing themselves to protect the other amoebae from bacteria is similar to the self-sacrifice by the phagocytes seen in the immune system of higher organisms. This innate immune function in social amoebae suggests an ancient cellular foraging mechanism that may have been adapted to defense functions well before the diversification of the animals.[84] The ability of amoebae to distinguish between self and non-self is a pivotal one that is also found in the immune system.[12]
References
- Notes
- ^ a b c d e f g h i j k l m n Mayer, Gene (2006). "Immunology — Chapter One: Innate (non-specific) Immunity". Microbiology and Immunology On-Line Textbook. USC School of Medicine. Retrieved 2008-11-12.
- ^ a b Schlenke TA, Morales J, Govind S, Clark AG (2007). "Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster". PLoS Pathog. 3 (10): 1486–501. doi:0.1371/journal.ppat.0030158. PMC 2042021. PMID 17967061. Retrieved 2009-02-23.
{{cite journal}}: Check|doi=value (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ The Shorter Oxford English Dictionary. Oxford University Press (Guild Publishing). 1983. pp. 1566–67.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Ernst p. 186
- ^ a b Robinson p. 187 and Ernst pp. 7–10
- ^ a b Ernst p. 10
- ^ a b Janeway, Charles A. (2007). Immunobiology: Antigen Presentation to T Lymphocytes. Garland Science. ISBN 978-0-8153-4123-9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "ATP" was defined multiple times with different content (see the help page). - ^ a b Lange, C (2007). "Dendritic cell-regulatory T-cell interactions control self-directed immunity". Immunology and cell biology. 85 (8): 575–81. PMID 17592494. Retrieved 2009-02-15.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Steinman, Ralph M. (2004). "Dendritic Cells and Immune Tolerance". The Rockefeller University. Retrieved 2009-02-15.
- ^ a b "Ilya Mechnikov". The Nobel Foundation. Retrieved 2008-11-28.
- ^ a b Schmalstieg, FC (2008). "Ilya Ilich Metchnikoff (1845-1915) and Paul Ehrlich (1854-1915): the centennial of the 1908 Nobel Prize in Physiology or Medicine". Journal of medical biography. 16 (2): 96–103. PMID 18463079. Retrieved 2008-11-28.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Janeway, Charles A. (2001). Immunobiology:Evolution of the innate immune system. Garland Science. ISBN 978-0-8153-4123-9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Robinson p. vii
- ^ "Carl Friedrich Claus, 1835–99". University of Cambridge. Retrieved 2009-2-15.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Aterman K (1998). "Medals, memoirs--and Metchnikoff". J. Leukoc. Biol. 63 (4): 515–7. PMID 9544583. Retrieved 2009-02-15.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Robinson p. vii
- ^ a b Hess, Charles. "Monocyte". University of Virginia Health System. Retrieved 2008-11-14. Cite error: The named reference "mono" was defined multiple times with different content (see the help page).
- ^ a b Hess, Charles. "Dendritic Cell". University of Virginia Health System. Retrieved 2008-11-14. Cite error: The named reference "dendrite" was defined multiple times with different content (see the help page).
- ^ Takahashi K, Naito M, Takeya M (1996). "Development and heterogeneity of macrophages and their related cells through their differentiation pathways". Pathol. Int. 46 (7): 473–85. PMID 8870002.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Hess, Charles. "Histiocyte". University of Virginia Health System. Retrieved 2008-11-14.
- ^ a b c d e Bowers, William (2006). "Immunology -Chapter Thirteen: Immunoregulation". Microbiology and Immunology On-Line Textbook. USC School of Medicine. Retrieved 2008-11-14. Cite error: The named reference "USCmac" was defined multiple times with different content (see the help page).
- ^ a b Alberts, Bruce (2002). Molecular Biology of the Cell; Fourth Edition. New York and London: Garland Science. ISBN 0-8153-3218-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ryter, A (1985). "Relationship between ultrastructure and specific functions of macrophages". Comparative Immunology, Microbiology and Infectious Diseases. 8 (2): 119–33. PMID 3910340. Retrieved 2008-11-14.
- ^ Hess, Charles. "Segmented Neutrophil". University of Virginia Health System. Retrieved 2008-11-14.
- ^ Paoletti p. 62
- ^ Soehnlein, O (2008). "Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages". Clinical and Experimental Immunology. 151 (1): 139–45. PMID 17991288. Retrieved 2008-11-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Soehnlein, O (2008). "Neutrophil primary granule proteins HBP and HNP1–3 boost bacterial phagocytosis by human and murine macrophages". The Journal of Clinical Investigation. 118 (10): 3491–502. PMID 18787642. Retrieved 2008-11-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Steinman RM, Cohn ZA (1973). "Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution". J. Exp. Med. 137 (5): 1142–62. doi:10.1084/jem.137.5.1142. PMID 4573839.
- ^ Sallusto F, Lanzavecchia A (2002). "The instructive role of dendritic cells on T-cell responses". Arthritis Res. 4 Suppl 3: S127–32. PMID 12110131.
- ^ a b Steinman, Ralph. "Dendritic Cells". The Rockefeller University. Retrieved 2008-11-16.
- ^ a b Guermonprez, P (2002). "Antigen presentation and T cell stimulation by dendritic cells". Annual Review of Immunology. 20: 621–67. PMID 11861614. Retrieved 2008-11-12.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Stelekati, E (2007). "Mast cells in allergy: innate instructors of adaptive responses". Immunobiology. 212 (6): 505–19. PMID 17544835. Retrieved 2009-02-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Malaviya, R (2001). "Mast cell modulation of immune responses to bacteria". Immunological reviews. 179: 16–24. PMID 11292019. Retrieved 2009-02-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Malaviya, R (1996). "Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells". Immunological reviews. 156 (4): 1490–6. PMID 8568252. Retrieved 2009-02-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Taylor, ML (2001). "Mast cells in allergy and host defense". Allergy and asthma proceedings: the official journal of regional and state allergy societies. 22 (3): 115–9. PMID 11424870. Retrieved 2009-02-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ J. Li, D.R. Barreda, Y.-A. Zhang, H. Boshra, A.E. Gelman, S. LaPatra, L. Tort & J.O. Sunyer (2006). "B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities". Nature Immunology. 7: 1116–1124. doi:10.1038/ni1389. PMID 16980980.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Paoletti p. 427
- ^ Birge RB, Ucker DS (2008). "Innate apoptotic immunity: the calming touch of death". Cell Death Differ. 15 (7): 1096–102. doi:10.1038/cdd.2008.58. PMID 18451871. Retrieved 2009-02-23.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S (2000). "Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes". Infect. Immun. 68 (12): 6939–45. PMC 97802. PMID 11083817. Retrieved 2009-02-22.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Paoletti p.426
- ^ May, RC (2001). "Phagocytosis and the actin cytoskeleton". Journal of Cell Science. 114 (6): 119–33. PMID 11228151. Retrieved 2008-11-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Ernst p. 4
- ^ a b Ryter, A (1985). "Relationship between ultrastructure and specific functions of macrophages". Comparative Immunology, Microbiology and Infectious Diseases. 8 (2): 119–33. PMID 3910340. Retrieved 2008-11-13.
- ^ Royet J (2004). "Infectious non-self recognition in invertebrates: lessons from Drosophila and other insect models". Mol. Immunol. 41 (11): 1063–75. doi:10.1016/j.molimm.2004.06.009. PMID 15476918. Retrieved 2009-02-13.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Janeway, Charles A. Induced innate responses to infection. ISBN 978-0-8153-4123-9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zen, K (2005). "Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils". Molecular Biology of the Cell. 16 (6): 2694–703. PMID 15800062. Retrieved 2008-11-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zen, K (2003). "Leukocyte-epithelial interactions". Current Opinion in Cell Biology. 15 (5): 557–64. PMID 14519390. Retrieved 2008-11-12.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Dale DC, Boxer L, Liles WC (2008). "The phagocytes: neutrophils and monocytes". Blood. 112 (4): 935–45. doi:10.1182/blood-2007-12-077917. PMID 18684880. Retrieved 2009-02-22.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dahlgren, C (1999-12-17). "Respiratory burst in human neutrophils". Journal of Immunological Methods. 232 (1–2): 3–14. PMID 10618505. Retrieved 2008-11-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Shatwell, KP (1996). "NADPH oxidase". The international journal of biochemistry and cell biology. 28 (11): 1191–5. PMID 9022278. Retrieved 2008-12-30.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Klebenoff, SJ (1999). "Myeloperoxidase". Proceedings of the Association of American Physicians. 111 (5): 383–9. PMID 10519157. Retrieved 2008-12-30.
- ^ Masek, Katherine S. (2007). Eurekah Bioscience Collection: Macrophage Effector Functions. Landes Bioscience.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Antigen Presenting Cells (APC)". Dalhousie University. Retrieved 2008-11-13.
- ^ Romagnani, S (2006). "Immunological tolerance and autoimmunity". Internal and emergency medicine. 1 (3): 187–96. PMID 17120464. Retrieved 2009-02-15.
- ^ Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA (2003). "Phosphatidylserine receptor is required for clearance of apoptotic cells". Science (journal). 302 (5650): 1560–3. doi:10.1126/science.1087621. PMID 14645847. Retrieved 2009-02-17.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wang X; et al. (2003). "Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12". Science. 302 (5650): 1563–1566. doi:10.1126/science.1087641. PMID 14645848.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Savill J, Gregory C, Haslett C. (2003). "Eat me or die". Science. 302 (5650): 1516–7. doi:10.1126/science.1092533. PMID 14645835.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zhou Z, Yu X (2008). "Phagosome maturation during the removal of apoptotic cells: receptors lead the way". Trends Cell Biol. 18 (10): 474–85. doi:10.1016/j.tcb.2008.08.002. PMID 18774293. Retrieved 2009-02-16.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e Todar, Kenneth. "Mechanisms of Bacterial Pathogenicity: Bacterial Defense Against Phagocytes". 2008. Retrieved 2008-12-10. Cite error: The named reference "chicken" was defined multiple times with different content (see the help page).
- ^ Celli J, Finlay BB (2002). "Bacterial avoidance of phagocytosis". Trends Microbiol. 10 (5): 232–7. PMID 11973157. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Burns, SM (1999). "Loss of resistance to ingestion and phagocytic killing by O(-) and K(-) mutants of a uropathogenic Escherichia coli O75:K5 strain". Infection and immunity. 67 (8): 3757–62. PMID 10417134. Retrieved 2008-12-10.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vuong, C (2004-12-24). "A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence". The journal of biological chemistry. 279 (52): 54881–6. PMID 15501828. Retrieved 2008-12-10.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Melin, M (2008-12-1). "Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B". Infection and immunity. PMID 19047408. Retrieved 2008-12-10.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Foster TJ (2005). "Immune evasion by staphylococci". Nat. Rev. Microbiol. 3 (12): 948–58. doi:10.1038/nrmicro1289. PMID 16322743. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help) Cite error: The named reference "pmid16322743" was defined multiple times with different content (see the help page). - ^ Sansonetti P (2001). "Phagocytosis of bacterial pathogens: implications in the host response". Semin. Immunol. 13 (6): 381–90. doi:10.1006/smim.2001.0335. PMID 11708894. Retrieved 2009-02-11.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Masek, Katherine S. (2007). Eurekah Bioscience Collection: Evasion of Phagosome Lysosome Fusion and Establishment of a Replicative Organelle by the Intracellular Pathogen Legionella pneumophila. Landes Bioscience.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Das, D (2008-12-1). "Intracellular survival of Staphylococcus aureus: correlating production of catalase and superoxide dismutase with levels of inflammatory cytokines". Infection and immunity. 57 (7): 340–9. PMID 18607538. Retrieved 2008-12-10.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hara H, Kawamura I, Nomura T, Tominaga T, Tsuchiya K, Mitsuyama M (2007). "Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement". Infect. Immun. 75 (8): 3791–801. doi:10.1128/IAI.01779-06. PMC 1951982. PMID 17517863. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Datta V, Myskowski SM, Kwinn LA, Chiem DN, Varki N, Kansal RG, Kotb M, Nizet V (2005). "Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection". Mol. Microbiol. 56 (3): 681–95. doi:10.1111/j.1365-2958.2005.04583.x. PMID 15819624. Retrieved 2008-12-13.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Iwatsuki K, Yamasaki O, Morizane S, Oono T (2006). "Staphylococcal cutaneous infections: invasion, evasion and aggression". J. Dermatol. Sci. 42 (3): 203–14. doi:10.1016/j.jdermsci.2006.03.011. PMID 16679003. Retrieved 2008-12-12.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Miller ME (1975). "Pathology of chemotaxis and random mobility". Semin. Hematol. 12 (1): 59–82. PMID 1088998.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help) - ^ Cline MJ, Lehrer RI, Territo MC, Golde DW (1978). "UCLA Conference. Monocytes and macrophages: functions and diseases". Ann. Intern. Med. 88 (1): 78–88. PMID 339803.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Roberts R, Gallin JI (1983). "The phagocytic cell and its disorders". Ann Allergy. 51 (3): 330–43. PMID 6311059.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help) - ^ Stein SM, Dale DC (2003). "Molecular basis and therapy of disorders associated with chronic neutropenia". Curr Allergy Asthma Rep. 3 (5): 385–8. PMID 12906773.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help) - ^ Goldman JM, Foroozanfar N, Gazzard BG, Hobbs JR (1984). "Lazy leukocyte syndrome". J R Soc Med. 77 (2): 140–1. PMC 1439703. PMID 6610763.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Speert DP (2006). "Bacterial infections of the lung in normal and immunodeficient patients". Novartis Found. Symp. 279: 42–51, disussion 51–5, 216–9. PMID 17278384.
{{cite journal}}:|access-date=requires|url=(help) - ^ Lipu HN, Ahmed TA, Ali S, Ahmed D, Waqar MA (2008). "Chronic granulomatous disease". J Pak Med Assoc. 58 (9): 516–8. PMID 18846805.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kaplan J, De Domenico I, Ward DM (2008). "Chediak-Higashi syndrome". Curr. Opin. Hematol. 15 (1): 22–9. doi:10.1097/MOH.0b013e3282f2bcce. PMID 18043242. Retrieved 2009-02-20.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Paoletti p. 426
- ^ Abraham E (2003). "Neutrophils and acute lung injury". Crit. Care Med. 31 (4 Suppl): S195–9. doi:10.1097/01.CCM.0000057843.47705.E8. PMID 12682440. Retrieved 2009-01-20.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lee WL, Downey GP (2001). "Neutrophil activation and acute lung injury". Curr Opin Crit Care. 7 (1): 1–7. PMID 11373504. Retrieved 2009-01-20.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Moraes TJ, Zurawska JH, Downey GP (2006). "Neutrophil granule contents in the pathogenesis of lung injury". Curr. Opin. Hematol. 13 (1): 21–7. PMID 16319683.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Heinzelmann M, Mercer-Jones MA, Passmore JC (1999). "Neutrophils and renal failure". Am. J. Kidney Dis. 34 (2): 384–99. doi:10.1053/AJKD03400384. PMID 10430993. Retrieved 2009-01-31.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chen G, Zhuchenko O, Kuspa A (2007). "Immune-like phagocyte activity in the social amoeba". Science (journal). 317 (5838): 678–81. doi:10.1126/science.1143991. PMID 17673666. Retrieved 2009-02-14.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
- Bibliography
- Ernst J. D. and Stendahl O., (editors), Phagocytosis of Bacteria and Bacterial Pathogenicity, Cambridge University Press, 2006, ISBN 0-521-84569-6 Website
- Paoletti R., Notario A. and Ricevuti G., (editors), Phagocytes: Biology, Physiology, Pathology, and Pharmacotherapeutics, The New York Academy of Sciences, 1997, ISBN 1-57331-102-2.
- Robinson J.P. and Babcock G. F., (editors), Phagocyte Function —A guide for research and clinical evaluation, Wiley–Liss, 1998, ISBN 0-471-12364-1.
External links
- Phagocytes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
