Subthalamic nucleus: Difference between revisions
| Line 26: | Line 26: | ||
===Afferent axons=== |
===Afferent axons=== |
||
The subthalamic nucleus receives its main input from the [[GPe|globus pallidus]] {{ |
The subthalamic nucleus receives its main input from the [[GPe|globus pallidus]] {{cite journal |author=Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA |title=Afferent connections of the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat |journal=Brain Res. |volume=513 |issue=1 |pages=43–59 |year=1990 |month=April |pmid=2350684 |doi= |url=}}, not so much through the ansa lenticularis as often said but by radiating fibers crossing the medial pallidum first and the internal capsule (see figure). This afference is GABAergic, inhibiting the neurons of the subthalamic nucleus. Excitatory, glutamatergic inputs come from the [[cerebral cortex]] (particularly the motor cortex), and from the pars [[parafascicularis]] of the [[central complex]]. The subthalamic nucleus also receives [[neuromodulator]]y inputs, notably [[dopaminergic]] axons from the [[substantia nigra]] pars compacta.<ref>{{cite journal | author=Cragg S.J.; Baufreton J.; Xue Y.; Bolam J.P.; & Bevan M.D. | title=Synaptic release of dopamine in the subthalamic nucleus | journal=European Journal of Neuroscience | year=2004 | pages=1788–1802 | volume=20 | issue=7 | pmid=15380000 | doi=10.1111/j.1460-9568.2004.03629.x}}</ref> |
||
It also receives inputs from the Pedunculopontine nucleus. |
It also receives inputs from the Pedunculopontine nucleus. |
||
Revision as of 02:19, 13 January 2010
| Subthalamic nucleus | |
|---|---|
 Coronal slices of human brain showing the basal ganglia, subthalamic nucleus (STN) and substantia nigra (SN). | |
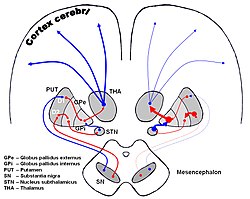 DA-loops in Parkinson's disease | |
| Details | |
| Part of | Basal ganglia |
| Identifiers | |
| Latin | nucleus subthalamicus |
| MeSH | D020531 |
| NeuroNames | 435 |
| NeuroLex ID | nlx_anat_1010002 |
| TA98 | A14.1.08.702 |
| TA2 | 5709 |
| FMA | 62035 |
| Anatomical terms of neuroanatomy | |
The subthalamic nucleus is a small lens-shaped nucleus in the brain where it is a part of the basal ganglia system. As suggested by its name, the subthalamic nucleus is located ventral to the thalamus. It is also dorsal to the substantia nigra and medial to the internal capsule. It was first described by Jules Bernard Luys in 1865,[1] and the term corpus Luysi or Luys' body is still sometimes used.
Anatomy
Structure
The principal type of neuron found in the subthalamic nucleus has rather long sparsely spiny dendrites.[2][3] The dendritic arborizations are ellipsoid, replicating in smaller dimension the shape of the nucleus.[4] The dimensions of these arborizations (1200,600 and 300 μm) are similar across many species—including rat, cat, monkey and man—which is unusual. However, the number of neurons increases across evolution as well as the external dimensions of the nucleus. Due to the bending of dendrites at the border, the subthalamic nucleus is a close nucleus, able to receive information only in its space. The principal neurons are glutamatergic, which give them a particular functional position in the basal ganglia system. In humans there are also a small number (about 7.5%) of GABAergic interneurons that participate in the local circuitry.[5]
Afferent axons
The subthalamic nucleus receives its main input from the globus pallidus Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA (1990). "Afferent connections of the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat". Brain Res. 513 (1): 43–59. PMID 2350684. {{cite journal}}: Unknown parameter |month= ignored (help)CS1 maint: multiple names: authors list (link), not so much through the ansa lenticularis as often said but by radiating fibers crossing the medial pallidum first and the internal capsule (see figure). This afference is GABAergic, inhibiting the neurons of the subthalamic nucleus. Excitatory, glutamatergic inputs come from the cerebral cortex (particularly the motor cortex), and from the pars parafascicularis of the central complex. The subthalamic nucleus also receives neuromodulatory inputs, notably dopaminergic axons from the substantia nigra pars compacta.[6]
It also receives inputs from the Pedunculopontine nucleus.
Efferent targets
The axons of subthalamic nucleus neurons leave the nucleus dorsally. The efferent axons are glutamatergic (excitatory). Except for the connection to the striatum (17.3% in macaques), most of the subthalamic principal neurons are multitargets and directed to the other elements of the core of the basal ganglia.[7] Some send axons to the substantia nigra medially and to the medial and lateral nuclei of the pallidum laterally (3-target, 21.3%). Some are 2-target with the lateral pallidum and the substantia nigra (2.7%) or the lateral pallidum and the medial (48%). Less are single target for the lateral pallidum. In the pallidum, subthalamic terminals end in bands parallel to the pallidal border.[8][9] When all axons reaching this target are added, the main afference of the subthalamic nucleus is, in 82.7% of the cases, clearly the medial pallidum (internal segment of the globus pallidus).
Some researchers have reported internal axon collaterals.[10] However, there is little functional evidence for this.
Physiology
Subthalamic nucleus
The first intracellular electrical recordings of subthalamic neurons were performed using sharp electrodes in a rat slice preparation (Nakanishi et al., 1987). In these recordings three key observations were made, all three of which have dominated subsequent reports of subthalamic firing properties. The first observation was that, in the absence of current injection or synaptic stimulation, the majority of cells were spontaneously firing. The second observation is that these cells are capable of transiently firing at very high frequencies. The third observation concerns non-linear behaviors when cells are transiently depolarized after being hyperpolarized below –65mV. They are then able to engage voltage-gated calcium and sodium currents to fire bursts of action potentials.
Several recent studies have focused on the autonomous pacemaking ability of subthalamic neurons. These cells are often referred to as "fast-spiking pacemakers",[11] since they can generate spontaneous action potentials at rates of 80 to 90Hz in primates.
Lateropallido-subthalamic system
Strong reciprocal connections link the subthalamic nucleus and the external segment of the globus pallidus. Both are fast-spiking pacemakers. Together, they are thought to constitute the "central pacemaker of the basal ganglia"[12] with synchronous bursts.
The connection of the lateral pallidum with the subthalamic nucleus is also the one in the basal ganglia system where the reduction between emitter/receiving elements is likely the strongest. In terms of volume, in humans, the lateral pallidum measures 808 mm³, the subthalamic nucleus only 158 mm³.[13] This translated in numbers of neurons represents a strong compression with loss of map precision.
Some axons from the lateral pallidum go to the striatum.[14] The activity of the medial pallidum is influenced by afferences from the lateral pallidum and from the subthalamic nucleus.[15] The same for the nigra reticulata.[9] The subthalamic nucleus sends axons to another regulator: the pedunculo-pontine complex (id).
The lateropallido-subthalamic system is thought to play a key role in the generation of the patterns of activity seen in Parkinson's disease.[16]
Physiopathology and interventions
Chronic stimulation of the STN, called deep brain stimulation (DBS), is used to treat patients with Parkinson disease. The first to be stimulated are the terminal arborisations of afferent axons which modifies the activity of subthalamic neurons. However, it has been shown in thalamic slices from mice[17], that the stimulus also causes nearby astrocytes to release Adenosine Triphosphate (ATP), a precursor to adenosine (through a catabolic process). In turn, adenosine A1 receptor activation depresses excitatory transmission in the thalamus, thus mimicking ablation of the subthalamic nucleus.
Pathology
Unilateral destruction or disruption of the subthalamic nucleus – which can commonly occur via a small vessel stroke in patients with diabetes, hypertension, or a history of smoking – produces hemiballismus.
Function
The function of the STN is unknown, but current theories place it as a component of the basal ganglia control system which may perform action selection. STN dysfunction has also been shown to increase impulsivity in individuals presented with two equally rewarding stimuli.[18]
Additional images
-
Coronal section of brain immediately in front of pons.
References
- ^ Luys, Jules Bernard (1865). Recherches sur le système cérébro-spinal, sa structure, ses fonctions et ses maladies (in French). Paris: Baillière.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/cne.902360102, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/cne.902360102instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/cne.901680105, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/cne.901680105instead. - ^ Yelnik, J. & Percheron, G. (1979). "Subthalamic neurons in primates : a quantitative and comparative anatomy". Neuroscience. 4 (11): 1717–1743. doi:10.1016/0306-4522(79)90030-7. PMID 117397.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Levesque J.C. & Parent A. (2005). "GABAergic interneurons in human subthalamic nucleus". Movement Disorders. 20 (5): 574–584. doi:10.1002/mds.20374. PMID 15645534.
- ^ Cragg S.J.; Baufreton J.; Xue Y.; Bolam J.P.; & Bevan M.D. (2004). "Synaptic release of dopamine in the subthalamic nucleus". European Journal of Neuroscience. 20 (7): 1788–1802. doi:10.1111/j.1460-9568.2004.03629.x. PMID 15380000.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cite error: The named reference
Sato2000was invoked but never defined (see the help page). - ^ Nauta, H.J.W. & Cole, M. (1978). "Efferent projections of the subthalamic nucleus : an autoradiographic study in monkey and cat". Journal of Comparative Neurology. 180 (1): 1–16. doi:10.1002/cne.901800102. PMID 418083.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Smith, Y.; Hazrati, L-N. & Parent, A. (1990). "Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method". Journal of Comparative Neurology. 294 (2): 306–323. doi:10.1002/cne.902940213. PMID 2332533.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kita, H.; Chang, H.T.; & Kitai, S.T. (1983). "The morphology of intracellularly labeled rat subthalamic neurons: A light microscopic analysis". Neuroscience. 215 (3): 245–257. PMID 6304154.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Surmeier D.J.; Mercer J.N.; & Chan C.S. (2005). "Autonomous pacemakers in the basal ganglia: who needs excitatory synapses anyway?". Current Opinion in Neurobiology. 15 (3): 312–318. doi:10.1016/j.conb.2005.05.007. PMID 15916893.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Plenz, D. & Kitai, S.T. (1999). "A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus". Nature. 400 (6745): 677–682. doi:10.1038/23281. PMID 10458164.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yelnik, J. (2002). "Functional anatomy of the basal ganglia". Movement Disorders. 17 (Suppl. 3): S15–S21. doi:10.1002/mds.10138. PMID 11948751.
- ^ Sato, F.; Lavallée, P.; Levesque, M. & Parent, A. (2000). "Single-axon tracing study of neurons of the external segment of the globus pallidus in primate". Journal of Comparative Neurology. 417 (1): 17–31. doi:10.1002/(SICI)1096-9861(20000131)417:1<17::AID-CNE2>3.0.CO;2-I. PMID 10660885.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Smith, Y.; Wichmann, T. & DeLong, M.R. (1994). "Synaptic innervation of neurones in the internal pallidal segment by the subthalamic nucleus and the external pallidum in monkeys". Journal of Comparative Neurology. 343 (2): 297–318. doi:10.1002/cne.903430209. PMID 8027445.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bevan M.D.; Magill P.J.; Terman D.; Bolam J.P.; & Wilson CJ. (2002). "Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network". Trends in Neurosciences. 25 (10): 525–531. doi:10.1016/S0166-2236(02)02235-X. PMID 12220881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bekar L., Libionka W., Tian G., Xu Q., Torres A., Wang X., Lovatt D., Williams E., Takano T., Schnermann J., Bakos R., Nedergaard M. (2008). "Adenosine is crucial for deep brain stimulation–mediated attenuation of tremor". Nature Medicine. 14 (1): 75–80. doi:10.1038/nm1693.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Frank, M.; Samanta, J.; Moustafa, A.; Sherman, S. (2007). "Hold Your Horses: Impulsivity, Deep Brain Stimulation, and Medication in Parkinsonism". Science. 318 (5854): 1309–12. doi:10.1126/science.1146157. PMID 17962524.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

