Allochronic speciation: Difference between revisions
Azcolvin429 (talk | contribs) full names |
Citation bot (talk | contribs) Alter: pages, issue, doi, year, pmid, pmc, url, title. URLs might have been internationalized/anonymized. Add: pmc, hdl, jstor, s2cid. Formatted dashes. | You can use this bot yourself. Report bugs here. | Suggested by Azcolvin429 | via #UCB_webform |
||
| Line 34: | Line 34: | ||
==== Isolation by time ==== |
==== Isolation by time ==== |
||
The concept of IBT warrants two probabilities: in the event that heritability in reproductive timing exists among populations that breed during different seasons, probability of mating will be, "inversely proportional to the difference in the heritable component of their reproductive times."<ref name="Hendry&Day2005"/><ref>{{Citation|title=Assortative mating and plant phenology: evolutionary and practical consequences |author=Gordon A. Fox |journal=Evolutionary Ecology Research |year=2003 |volume=5 |issue= |pages= |
The concept of IBT warrants two probabilities: in the event that heritability in reproductive timing exists among populations that breed during different seasons, probability of mating will be, "inversely proportional to the difference in the heritable component of their reproductive times."<ref name="Hendry&Day2005"/><ref>{{Citation|title=Assortative mating and plant phenology: evolutionary and practical consequences |author=Gordon A. Fox |journal=Evolutionary Ecology Research |year=2003 |volume=5 |issue= |pages=1–18 |url=http://www.evolutionary-ecology.com/issues/v05n01/ccar1383.pdf }}</ref> The probability of mating can also be proportional to breeding values (phenotypic trait expressed as the trait of tis offspring) for reproductive time in the event the heritability is [[Additive genetic effects|additive]] (more than one gene controls the phenotypic trait).<ref name="Hendry&Day2005"/> In a population, offspring will inherit the traits for reproductive time causing a decrease in gene flow while reproductive timing differences increase.<ref name="Hendry&Day2005"/> |
||
==== Adaptation by time ==== |
==== Adaptation by time ==== |
||
| Line 131: | Line 131: | ||
|- |
|- |
||
|''[[Exapion ulicis]]'' and ''[[Exapion lemovicinum|E. lemovicinum]]'' |
|''[[Exapion ulicis]]'' and ''[[Exapion lemovicinum|E. lemovicinum]]'' |
||
|''E. lemovicinum'' infects ''[[Ulex minor]]'' and ''[[Ulex gallii|U. gallii]]'' plants while ''E. ulicis'' infects ''[[Ulex europaeus|U. europaeus]]''. The timing in which they lay eggs on the plant occurs in fall and spring respectively.<ref>{{Citation|title=Plant phenology and seed predation: Interactions between gorses and weevils in Brittany (France) |author= Barat M, Tarayre M, and Atlan A |journal=Entomologia Experimentalis et Applicata |year=2007 |volume=124 |issue= |pages=167–176 |doi= |pmid= |url= }}</ref> |
|''E. lemovicinum'' infects ''[[Ulex minor]]'' and ''[[Ulex gallii|U. gallii]]'' plants while ''E. ulicis'' infects ''[[Ulex europaeus|U. europaeus]]''. The timing in which they lay eggs on the plant occurs in fall and spring respectively.<ref>{{Citation|title=Plant phenology and seed predation: Interactions between gorses and weevils in Brittany (France) |author= Barat M, Tarayre M, and Atlan A |journal=Entomologia Experimentalis et Applicata |year=2007 |volume=124 |issue= 2|pages=167–176 |doi= 10.1111/j.1570-7458.2007.00565.x|pmid= |s2cid= 85880513 |url= }}</ref> |
||
|- |
|- |
||
|''[[Meconopsis autumnalis]]'' and ''[[Meconopsis paniculata|M. paniculata]]'' |
|''[[Meconopsis autumnalis]]'' and ''[[Meconopsis paniculata|M. paniculata]]'' |
||
|Himalayan poppy are a fully reproductively isolated species thought to have speciated through allochrony as they exist in sympatry and flower at different times in the season.<ref>{{Citation|title=''Meconopsis autumnalis'' and ''M. manasluensis'' (Papaveraceae), two new species of Himalayan poppy endemic to central Nepal with sympatric congeners |author= Egan PA |journal=Phytotaxa |year= |volume=20 |issue= |pages=47–56 |doi= |pmid= |url= }}</ref> |
|Himalayan poppy are a fully reproductively isolated species thought to have speciated through allochrony as they exist in sympatry and flower at different times in the season.<ref>{{Citation|title=''Meconopsis autumnalis'' and ''M. manasluensis'' (Papaveraceae), two new species of Himalayan poppy endemic to central Nepal with sympatric congeners |author= Egan PA |journal=Phytotaxa |year= 2011|volume=20 |issue= |pages=47–56 |doi= 10.11646/phytotaxa.20.1.4|pmid= |url= }}</ref> |
||
|- |
|- |
||
|''[[Cordia]]'' spp. |
|''[[Cordia]]'' spp. |
||
|Some of the species in the genus exhibit significant variation in flowering times.<ref>{{Citation|title=Reproductive biology of some Costa Rican ''Cordia'' species (Boraginaceae) |author=Opler PA, Baker HG, and Frankie GW |journal=Biotropica |year=1975 |volume=7 |issue= |pages= |
|Some of the species in the genus exhibit significant variation in flowering times.<ref>{{Citation|title=Reproductive biology of some Costa Rican ''Cordia'' species (Boraginaceae) |author=Opler PA, Baker HG, and Frankie GW |journal=Biotropica |year=1975 |volume=7 |issue= |pages=234–247 |doi= 10.2307/2989736|jstor=2989736 |pmid= |url= }}</ref> |
||
|- |
|- |
||
|[[Skipper (butterfly)|Hesperiidae]] |
|[[Skipper (butterfly)|Hesperiidae]] |
||
|It is thought that temporal isolation is responsible for speciation in many of the 400 skipper butterfly species studied.<ref>{{Citation|title=Diel activity and reproductive isolation in a diverse assemblage of Neotropical skippers (Lepidoptera: Hesperiidae) |author= Devries PJ, Austin GT, and Martin NH |journal=Biological Journal of the Linnean Society |year=2008 |volume=94 |issue= |pages= |
|It is thought that temporal isolation is responsible for speciation in many of the 400 skipper butterfly species studied.<ref>{{Citation|title=Diel activity and reproductive isolation in a diverse assemblage of Neotropical skippers (Lepidoptera: Hesperiidae) |author= Devries PJ, Austin GT, and Martin NH |journal=Biological Journal of the Linnean Society |year=2008 |volume=94 |issue= 4|pages=723–736 |doi= 10.1111/j.1095-8312.2008.01037.x|pmid= |url= }}</ref> |
||
|- |
|- |
||
|''[[Bryopsidales]]'' spp. |
|''[[Bryopsidales]]'' spp. |
||
|The green algae reproduces by releasing gametes at different times. This is thought to have driven reproductive isolation, but it is unclear if it is genetically controlled or based purely on environmental cues.<ref>{{Citation|title=The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs |author=Clifton K and Clifton L |journal=Journal of Phycology |year=1999 |volume=35 |issue= |pages=24–34 |doi= |pmid= |url= }}</ref> |
|The green algae reproduces by releasing gametes at different times. This is thought to have driven reproductive isolation, but it is unclear if it is genetically controlled or based purely on environmental cues.<ref>{{Citation|title=The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs |author=Clifton K and Clifton L |journal=Journal of Phycology |year=1999 |volume=35 |issue= |pages=24–34 |doi= 10.1046/j.1529-8817.1999.3510024.x|pmid= |s2cid=83704320 |url= }}</ref> |
||
|- |
|- |
||
|''[[Chilo suppressalis]]'' |
|''[[Chilo suppressalis]]'' |
||
|Mate timing occurs at different intervals at night as well as dependence on different host plants.<ref>{{Citation|title=Difference in the time of mating activity between host-associated populations of the rice stem borer, ''Chilo suppressalis'' (Walker) |author=Ueno H, Furukawa S, and Tsuchida K |journal=Entomological Science |year=2006 |volume=9 |issue= |pages=255–259 |doi= |pmid= |url= }}</ref> |
|Mate timing occurs at different intervals at night as well as dependence on different host plants.<ref>{{Citation|title=Difference in the time of mating activity between host-associated populations of the rice stem borer, ''Chilo suppressalis'' (Walker) |author=Ueno H, Furukawa S, and Tsuchida K |journal=Entomological Science |year=2006 |volume=9 |issue= 3|pages=255–259 |doi= 10.1111/j.1479-8298.2006.00171.x|pmid= |s2cid=86106927 |url= }}</ref> |
||
|- |
|- |
||
|''[[Prodoxus quinquepunctellus]]'' |
|''[[Prodoxus quinquepunctellus]]'' |
||
|Host races of the moths inhabit [[Yucca filamentosa|Adam’s needle and thread yucca]] with larval emergence occurring in conjunction with flowering time. It is thought that morphology and host-shifting contribute alongside allochrony.<ref>{{Citation|title=Rapid evolution and specialization following host colonization in a yucca moth |author=Groman JD and Pellmyr O |journal=Journal of Evolutionary Biology |year=2000 |volume=13 |issue= |pages=223–236 |doi= |pmid= |url= }}</ref> |
|Host races of the moths inhabit [[Yucca filamentosa|Adam’s needle and thread yucca]] with larval emergence occurring in conjunction with flowering time. It is thought that morphology and host-shifting contribute alongside allochrony.<ref>{{Citation|title=Rapid evolution and specialization following host colonization in a yucca moth |author=Groman JD and Pellmyr O |journal=Journal of Evolutionary Biology |year=2000 |volume=13 |issue= 2|pages=223–236 |doi= 10.1046/j.1420-9101.2000.00159.x|pmid= |s2cid=84556390 |url= }}</ref> |
||
|- |
|- |
||
|''[[Gryllus pennsylvanicus]]'' and ''[[Gryllus veletis|G. veletis]]'' |
|''[[Gryllus pennsylvanicus]]'' and ''[[Gryllus veletis|G. veletis]]'' |
||
|The spring field crickets have been described as speciating in allochrony due to their maturation timing.<ref>{{Citation|title=Allochronic speciation in field crickets, and a new species, ''Acheta veletis'' |author=Alexander RD and Bigelow RS |journal=Evolution |year=1960 |volume=14 |issue= |pages=334–346 |doi= |pmid= |url= }}</ref> However more recent studies indicate that they are not sister species.<ref>{{Citation|title=Mitochondrial DNA phylogeny of North American field crickets: perspectives on the evolution of life cycles, songs, and habitat associations |author=Harrison RG, Bogdanowicz SM, and Hall C |journal=Journal of Evolutionary Biology |year=1995 |volume=8 |issue= |pages= |
|The spring field crickets have been described as speciating in allochrony due to their maturation timing.<ref>{{Citation|title=Allochronic speciation in field crickets, and a new species, ''Acheta veletis'' |author=Alexander RD and Bigelow RS |journal=Evolution |year=1960 |volume=14 |issue= 3|pages=334–346 |doi= 10.1111/j.1558-5646.1960.tb03095.x|pmid= |hdl=2027.42/137466 |s2cid=87867996 |url= }}</ref> However more recent studies indicate that they are not sister species.<ref>{{Citation|title=Mitochondrial DNA phylogeny of North American field crickets: perspectives on the evolution of life cycles, songs, and habitat associations |author=Harrison RG, Bogdanowicz SM, and Hall C |journal=Journal of Evolutionary Biology |year=1995 |volume=8 |issue= 2|pages=209–232 |doi= 10.1046/j.1420-9101.1995.8020209.x|pmid= |s2cid=85777010 |url= }}</ref> |
||
|- |
|- |
||
|''[[Haemaphysalis]]'' spp. and ''[[Dermacentor]]'' spp. |
|''[[Haemaphysalis]]'' spp. and ''[[Dermacentor]]'' spp. |
||
|Three Hungarian tick species in the ''Haemaphysalis'' genus exhibit mating activity in sympatry during three seasonal periods, late fall, late spring, and early spring. Two tick species in the ''Dermacentor'' genus show peak activity in fall and spring.<ref>{{Citation|title=Allochronic seasonal peak activities of ''Dermacentor'' and ''Haemaphysalis'' spp. under continental climate in Hungary |author=Hornok, S. |journal=Veterinary Parasitology |year=2009 |volume=163 |issue= |pages=366–369 |doi= |pmid= |url= }}</ref> |
|Three Hungarian tick species in the ''Haemaphysalis'' genus exhibit mating activity in sympatry during three seasonal periods, late fall, late spring, and early spring. Two tick species in the ''Dermacentor'' genus show peak activity in fall and spring.<ref>{{Citation|title=Allochronic seasonal peak activities of ''Dermacentor'' and ''Haemaphysalis'' spp. under continental climate in Hungary |author=Hornok, S. |journal=Veterinary Parasitology |year=2009 |volume=163 |issue= 4|pages=366–369 |doi= 10.1016/j.vetpar.2009.03.048|pmid= 19410373|url= }}</ref> |
||
|- |
|- |
||
|''[[Strauzia longipennis]]'' |
|''[[Strauzia longipennis]]'' |
||
|Genetic variation is detected in three sunflower maggot fly variants that inhabit the same host plant. Their larval emergence occurs in three distinct periods of the summer keeping them partially isolated. Experimental manipulation suggests allochrony will increase as they continue to diverge.<ref>{{Citation|title=Divergence before the host shift? Prezygotic reproductive isolation among three varieties of a specialist fly on a single host plant |author= Hippee AC, Elnes ME, Armenta JS, Condon MA, and Forbes AA |journal=Ecological Entomology |year=2016 |volume=41 |issue= |pages= |
|Genetic variation is detected in three sunflower maggot fly variants that inhabit the same host plant. Their larval emergence occurs in three distinct periods of the summer keeping them partially isolated. Experimental manipulation suggests allochrony will increase as they continue to diverge.<ref>{{Citation|title=Divergence before the host shift? Prezygotic reproductive isolation among three varieties of a specialist fly on a single host plant |author= Hippee AC, Elnes ME, Armenta JS, Condon MA, and Forbes AA |journal=Ecological Entomology |year=2016 |volume=41 |issue= 4|pages=389–399 |doi= 10.1111/een.12309|pmid= |s2cid= 87320676 |url= }}</ref><ref>{{Citation|title=Genetically differentiated races and speciation-with-gene-flow in the sunflower maggot, ''Strauzia longipennis'' |author= Forbes AA, Kelly PH, Middleton KA, and Condon MA |journal=Evolutionary Ecology |year=2013 |volume=27 |issue= 5|pages=1017–1032 |doi= 10.1007/s10682-012-9622-y|pmid= |s2cid= 18472327 |url= }}</ref> |
||
|- |
|- |
||
|[[Enchenopa binotata|''Enchenopa binotata'' complex]] |
|[[Enchenopa binotata|''Enchenopa binotata'' complex]] |
||
|The phenology of members in the treehopper species complex is correlated with their host plants—when changing host species in experiment, the treehopper egg hatching time changes promoting assortive mating.<ref>{{Citation|title=Insect phenology mediated by host-plant water relations |author= Wood TK, Olmstead KL, and Guttman SI |journal=Evolution |year=1990 |volume=44 |issue= |pages= |
|The phenology of members in the treehopper species complex is correlated with their host plants—when changing host species in experiment, the treehopper egg hatching time changes promoting assortive mating.<ref>{{Citation|title=Insect phenology mediated by host-plant water relations |author= Wood TK, Olmstead KL, and Guttman SI |journal=Evolution |year=1990 |volume=44 |issue= 3|pages=629–636 |doi= 10.1111/j.1558-5646.1990.tb05943.x|pmid= 28567982|s2cid= 43689173 |url= }}</ref> |
||
|- |
|- |
||
|''[[Milicia excelsa]]'' |
|''[[Milicia excelsa]]'' |
||
|With flowering time of the African teak thought to be genetically controlled, the population exhibits isolation.<ref>{{Citation|title=Phenological patterns in a natural population of a tropical timber tree species, ''Milicia excelsa'' (Moraceae): Evidence of isolation by time and its interaction with feeding strategies of dispersers |author=Kasso Daïnou, Eric Laurenty, Grégory Mahy, Olivier J Hardy, Yves Brostaux, Nikki Tagg, and Jean-Louis Doucet |journal=American Journal of Botany |year=2012 |volume=99 |issue= |pages=1453–1463 |doi=10.3732/ajb.1200147 |pmid=22912370 |url=https://bsapubs.onlinelibrary.wiley.com/doi/pdf/10.3732/ajb.1200147 }}</ref> |
|With flowering time of the African teak thought to be genetically controlled, the population exhibits isolation.<ref>{{Citation|title=Phenological patterns in a natural population of a tropical timber tree species, ''Milicia excelsa'' (Moraceae): Evidence of isolation by time and its interaction with feeding strategies of dispersers |author=Kasso Daïnou, Eric Laurenty, Grégory Mahy, Olivier J Hardy, Yves Brostaux, Nikki Tagg, and Jean-Louis Doucet |journal=American Journal of Botany |year=2012 |volume=99 |issue= 9|pages=1453–1463 |doi=10.3732/ajb.1200147 |pmid=22912370 |url=https://bsapubs.onlinelibrary.wiley.com/doi/pdf/10.3732/ajb.1200147 }}</ref> |
||
|- |
|- |
||
|''[[Asteralobia sasakii]]'' |
|''[[Asteralobia sasakii]]'' |
||
|Two populations of [[Cecidomyiidae|Cecidomyiidae gall midges]] differ substantially in emergence time (with no overlap) on two different ''[[Ilex]]'' hosts. <ref>{{Citation|title=Host-associated differences in emergence pattern, reproductive behavior and life history of ''Asteralobia sasakii'' (Monzen)(Diptera: Cecidomyiidae) between populations on ''Ilex crenata'' and ''I. integra'' (Aquifoliaceae) |author=Tabuchi K, Amano H |journal=Applied Entomology and Zoology |year=2003 |volume=38 |issue= |pages= |
|Two populations of [[Cecidomyiidae|Cecidomyiidae gall midges]] differ substantially in emergence time (with no overlap) on two different ''[[Ilex]]'' hosts. <ref>{{Citation|title=Host-associated differences in emergence pattern, reproductive behavior and life history of ''Asteralobia sasakii'' (Monzen)(Diptera: Cecidomyiidae) between populations on ''Ilex crenata'' and ''I. integra'' (Aquifoliaceae) |author=Tabuchi K, Amano H |journal=Applied Entomology and Zoology |year=2003 |volume=38 |issue= |pages=501–508 |doi= 10.1303/aez.2003.501|pmid= |url= }}</ref> |
||
|- |
|- |
||
|''[[Salix]]'' spp. |
|''[[Salix]]'' spp. |
||
|The Canadian willow species are found to isolated by flowering time; three of which flower early (''[[Salix bebbiana|S. bebbiana]]'', ''[[Salix discolor|S. discolor]]'', ''[[Salix eriocephala|S. eriocephala]]'', and ''[[Salix petiolaris|S. petiolaris]]'') and late (''[[Salix amygdaloides|S. amygdaloides]]'', ''[[Salix exigua|S. exigua]]'', and ''[[Salix lucida|S. lucida]]''). Hybrids are not known outside of laboratory settings and exhibit intermediate flowering times. All seven species exist in sympatric distributions.<ref>{{Citation|title=Seasonal isolation as a reproductive barrier among sympatric ''Salix'' species |author=A. Mosseler and C. S. Papadopol |journal=Canadian Journal of Botany |year=1989 |volume=67 |issue= |pages=2563–2570 |doi=10.1139/b89-331 |pmid= |url= }}</ref> |
|The Canadian willow species are found to isolated by flowering time; three of which flower early (''[[Salix bebbiana|S. bebbiana]]'', ''[[Salix discolor|S. discolor]]'', ''[[Salix eriocephala|S. eriocephala]]'', and ''[[Salix petiolaris|S. petiolaris]]'') and late (''[[Salix amygdaloides|S. amygdaloides]]'', ''[[Salix exigua|S. exigua]]'', and ''[[Salix lucida|S. lucida]]''). Hybrids are not known outside of laboratory settings and exhibit intermediate flowering times. All seven species exist in sympatric distributions.<ref>{{Citation|title=Seasonal isolation as a reproductive barrier among sympatric ''Salix'' species |author=A. Mosseler and C. S. Papadopol |journal=Canadian Journal of Botany |year=1989 |volume=67 |issue= 9|pages=2563–2570 |doi=10.1139/b89-331 |pmid= |url= }}</ref> |
||
|- |
|- |
||
|''[[Juncus effusus]]'' |
|''[[Juncus effusus]]'' |
||
|Sympatric populations of genetically differentiated plants flower at different times preventing hybridization. It is unclear if speciation is occurring by allochrony as reinforcement may be a stronger explanation.<ref>{{Citation|title=Separation in flowering time contributes to the maintenance of sympatric cryptic plant lineages |author=Stefan G Michalski and Walter Durka |journal=Ecology and Evolution |year=2015 |volume=5 |issue=11 |pages=2172–2184 |doi=10.1002/ece3.1481 |pmid=26078854 |pmc= |
|Sympatric populations of genetically differentiated plants flower at different times preventing hybridization. It is unclear if speciation is occurring by allochrony as reinforcement may be a stronger explanation.<ref>{{Citation|title=Separation in flowering time contributes to the maintenance of sympatric cryptic plant lineages |author=Stefan G Michalski and Walter Durka |journal=Ecology and Evolution |year=2015 |volume=5 |issue=11 |pages=2172–2184 |doi=10.1002/ece3.1481 |pmid=26078854 |pmc=4461419 |url= }}</ref> |
||
|- |
|- |
||
|''[[Agrostis tenuis]]'' |
|''[[Agrostis tenuis]]'' |
||
| Line 182: | Line 182: | ||
|- |
|- |
||
|''[[Chironomus nuditarsis]]'' |
|''[[Chironomus nuditarsis]]'' |
||
|The non-biting midge (genus ''[[Chironomus]]'') exhibits differences in life cycle in accordance with elevation.<ref>{{Citation|title=Microevolutionary changes in populations of ''Chironomus nuditarsis'' Str. (Keyl, 1962) (Chironomidae, Diptera) from central Caucasus |author=Polukonova NV and Karmokov MK |journal=Russian Journal of Genetics |year=2013 |volume=49 |issue= |pages=175–181 |doi= |pmid= |url= }}</ref> |
|The non-biting midge (genus ''[[Chironomus]]'') exhibits differences in life cycle in accordance with elevation.<ref>{{Citation|title=Microevolutionary changes in populations of ''Chironomus nuditarsis'' Str. (Keyl, 1962) (Chironomidae, Diptera) from central Caucasus |author=Polukonova NV and Karmokov MK |journal=Russian Journal of Genetics |year=2013 |volume=49 |issue= 2|pages=175–181 |doi= 10.1134/S1022795413020099|pmid= |s2cid=12200060 |url= }}</ref> |
||
|- |
|- |
||
|''[[Terellia fuscicornis]]'' |
|''[[Terellia fuscicornis]]'' |
||
|Differences in courtship behavior as well as morphology are found in populations that infect different hosts (''[[Silybum marianum]]''and ''[[Cynara]]'') that bloom at different times.<ref>{{Citation|title=''Terellia fuscicornis'' (Diptera: Tephritidae): biological and morphological adaptation on artichoke and milk thistle |author= Sayar NP, Smith CA, White IM, Knio KM |journal=Journal of Natural History |year=2009 |volume=43 |issue= |pages=1159–1181 |doi= |pmid= |url= }}</ref> |
|Differences in courtship behavior as well as morphology are found in populations that infect different hosts (''[[Silybum marianum]]''and ''[[Cynara]]'') that bloom at different times.<ref>{{Citation|title=''Terellia fuscicornis'' (Diptera: Tephritidae): biological and morphological adaptation on artichoke and milk thistle |author= Sayar NP, Smith CA, White IM, Knio KM |journal=Journal of Natural History |year=2009 |volume=43 |issue= 19–20|pages=1159–1181 |doi= 10.1080/00222930902807742|pmid= |s2cid= 56004378 |url= }}</ref> |
||
|- |
|- |
||
|''[[Ampelomyces]]'' spp. |
|''[[Ampelomyces]]'' spp. |
||
|Genetically different strains of the [[Mycoparasitism|mycoparasitic]] fungus that infects apple powdery mildew completes their lifecycle before other strains that infect other mildew hosts.<ref>{{Citation|title=Temporal isolation explains host-related genetic differentiation in a group of widespread mycoparasitic fungi |author=Levente Kiss, Alexandra Pintye, Gábor M Kovács, Tünde Jankovics, Michael C Fontaine, Nick Harvey, Xiangming Xu, Philippe C Nicot, Marc Bardin, Jacqui A Shykoff, and Tatiana Giraud |journal=Molecular Ecology |year=2011 |volume=20 |issue=7 |pages=1492–1507 |doi=10.1111/j.1365-294X.2011.05007.x |pmid=21261766 |url= }}</ref> |
|Genetically different strains of the [[Mycoparasitism|mycoparasitic]] fungus that infects apple powdery mildew completes their lifecycle before other strains that infect other mildew hosts.<ref>{{Citation|title=Temporal isolation explains host-related genetic differentiation in a group of widespread mycoparasitic fungi |author=Levente Kiss, Alexandra Pintye, Gábor M Kovács, Tünde Jankovics, Michael C Fontaine, Nick Harvey, Xiangming Xu, Philippe C Nicot, Marc Bardin, Jacqui A Shykoff, and Tatiana Giraud |journal=Molecular Ecology |year=2011 |volume=20 |issue=7 |pages=1492–1507 |doi=10.1111/j.1365-294X.2011.05007.x |pmid=21261766 |s2cid=34557058 |url= https://hal.archives-ouvertes.fr/hal-02329042/file/45580_20110406104614260_2.pdf}}</ref> |
||
|- |
|- |
||
|''[[Glycine max]]'' and ''[[Glycine soja|G. soja]]'' |
|''[[Glycine max]]'' and ''[[Glycine soja|G. soja]]'' |
||
|Wild soybean (''G. soja'') and cultivated soybean (''G. max'') can be prevented from hybridizing by inducing asynchrony in flowering time.<ref>{{Citation|title=A new method for evaluating flowering synchrony to support the temporal isolation of genetically modified crops from their wild relatives |author=Ohigashi K, Mizuguti A, Yoshimura Y, Matsuo K, and Miwa T |journal=Journal of Plant Research |year=2014 |volume=127 |issue= |pages=109–117 |doi= |pmid= |url= }}</ref> This study is unique in that it is not an example of allochronic speciation, but instead an experiment demonstrating that allochrony can be experimentally applied to induce isolation. |
|Wild soybean (''G. soja'') and cultivated soybean (''G. max'') can be prevented from hybridizing by inducing asynchrony in flowering time.<ref>{{Citation|title=A new method for evaluating flowering synchrony to support the temporal isolation of genetically modified crops from their wild relatives |author=Ohigashi K, Mizuguti A, Yoshimura Y, Matsuo K, and Miwa T |journal=Journal of Plant Research |year=2014 |volume=127 |issue= 1|pages=109–117 |doi= 10.1007/s10265-013-0592-0|pmid= 24122370|s2cid=13606001 |url= }}</ref> This study is unique in that it is not an example of allochronic speciation, but instead an experiment demonstrating that allochrony can be experimentally applied to induce isolation. |
||
|- |
|- |
||
|Parasitoid wasps: ''[[Rhagoletis pomonella]]'', ''[[Rhagoletis mendax]]'', ''[[Diachasma alloeum]]'', ''[[Diachasmimorpha mellea]]'', and ''[[Utetes canaliculatus]]'' |
|Parasitoid wasps: ''[[Rhagoletis pomonella]]'', ''[[Rhagoletis mendax]]'', ''[[Diachasma alloeum]]'', ''[[Diachasmimorpha mellea]]'', and ''[[Utetes canaliculatus]]'' |
||
|In ''R. pomonella'' (one of the most researched, model organisms), genetic data indicates heritability of emergence and its associated flight time.<ref>{{Citation|title=Experimental evidence of genome-wide impact of ecological selection during early stages of speciation-with-gene-flow |author=Scott P Egan, Gregory J Ragland, Lauren Assour, Thomas H Q Powell, Glen R Hood, Scott Emrich, Patrik Nosil, and Jeffrey L Feder |journal=Ecology Letters |year=2015 |volume=18 |issue=8 |pages= |
|In ''R. pomonella'' (one of the most researched, model organisms), genetic data indicates heritability of emergence and its associated flight time.<ref>{{Citation|title=Experimental evidence of genome-wide impact of ecological selection during early stages of speciation-with-gene-flow |author=Scott P Egan, Gregory J Ragland, Lauren Assour, Thomas H Q Powell, Glen R Hood, Scott Emrich, Patrik Nosil, and Jeffrey L Feder |journal=Ecology Letters |year=2015 |volume=18 |issue=8 |pages=817–825 |doi=10.1111/ele.12460 |pmid=26077935 |pmc=4744793 |url= }}</ref> In commercial [[blueberry]] fields versus wild ones, the populations of ''R. mendax'' differ in their flight periods causing a reduction in gene flow.<ref>{{Citation|title=Evolution of phenologically distinct populations of ''Rhagoletis mendax'' (Diptera: Tephritidae) in Highbush Blueberry Fields |author=Teixeira LAF and Polavarapu S |journal=Annals of the Entomological Society of America |year=2003 |volume=96 |issue= 6|pages=818–827 |doi= 10.1603/0013-8746(2003)096[0818:EOPDPO]2.0.CO;2|pmid= |url= }}</ref> Other ''Rhagoletis'' species that host on ''[[Crataegus]]'' show similar patterns.<ref>{{Citation|title=Ecological adaptation and reproductive isolation in sympatry: Genetic and phenotypic evidence for native host races of ''Rhagoletis pomonella'' |author=Thomas H Q Powell, Andrew A Forbes, Glen R Hood, and Jeffrey L Feder |journal=Molecular Ecology |year=2014 |volume=23 |issue=3 |pages=688–704 |doi=10.1111/mec.12635 |pmid= 24351094 |s2cid=2745741 |url= }}</ref> [[Cospeciation]] of the parasitoid wasps (''D. alloeum'', ''D. mellea'', and ''U. canaliculatus'') and their host plant [[apple maggot]] has been induced by host-shifts caused by various factors such as timing of its egg hatching, fruit smell preference, [[philopatry]], and [[Avoidance response|avoidance]]. The egg hatching timing factor implicates allochrony.<ref>{{Citation|title=Sequential divergence and the multiplicative origin of community diversity |author=Glen R Hood, Andrew A Forbes, Thomas H Q Powell, Scott P Egan, Gabriela Hamerlinck, James J Smith, and Jeffrey L Feder |journal=Proceedings of the National Academy of Sciences |year=2015 |volume=112 |issue=44 |pages= E5980–E5989|doi=10.1073/pnas.1424717112 |pmid= 26499247 |pmc=4640724 |url= }}</ref> |
||
|- |
|- |
||
|''[[Eurosta solidaginis]]'' |
|''[[Eurosta solidaginis]]'' |
||
|Two populations of goldenrod gall fly differ in their emergence periods on their host plants ''[[Solidago altissima]]'' and ''[[Solidago gigantea|S. gigantea]]'' by 10 to 14 days preventing hybridization and causing isolation.<ref>{{Citation|title=Genetics, experience, and host-plant preference in ''Eurosta solidaginis'': implications for host shifts and speciation |author=Craig TP, Horner JD, and Itami JK |journal=Evolution |year=2001 |volume=55 |issue= |pages= |
|Two populations of goldenrod gall fly differ in their emergence periods on their host plants ''[[Solidago altissima]]'' and ''[[Solidago gigantea|S. gigantea]]'' by 10 to 14 days preventing hybridization and causing isolation.<ref>{{Citation|title=Genetics, experience, and host-plant preference in ''Eurosta solidaginis'': implications for host shifts and speciation |author=Craig TP, Horner JD, and Itami JK |journal=Evolution |year=2001 |volume=55 |issue= 4|pages=773–782 |doi= 10.1554/0014-3820(2001)055[0773:GEAHPP]2.0.CO;2|pmid= 11392395|url= }}</ref> |
||
|- |
|- |
||
|''[[Falco sparverius]]'' |
|''[[Falco sparverius]]'' |
||
|Kestrels of Idaho have both migrating and year-round residents with the year-round population nesting earlier generating assortive mating.<ref>{{Citation|title=Nesting phenology, mate choice, and genetic divergence within a partially migratory population of American Kestrels |author=Anderson AM, Novak SJ, Smith JF, Steenhof K, Heath JA |journal=The Auk |year= |volume=133 |issue= |pages=99–109 |doi= |pmid= |url= }}</ref> |
|Kestrels of Idaho have both migrating and year-round residents with the year-round population nesting earlier generating assortive mating.<ref>{{Citation|title=Nesting phenology, mate choice, and genetic divergence within a partially migratory population of American Kestrels |author=Anderson AM, Novak SJ, Smith JF, Steenhof K, Heath JA |journal=The Auk |year= 2016|volume=133 |issue= |pages=99–109 |doi= 10.1642/AUK-15-129.1|pmid= |s2cid=85683278 |url= }}</ref> |
||
|- |
|- |
||
|''[[Sylvia atricapilla]]'' |
|''[[Sylvia atricapilla]]'' |
||
| Line 206: | Line 206: | ||
|- |
|- |
||
|''[[Junco hyemalis]] hyemalis'' and ''J. h. carolinensis'' |
|''[[Junco hyemalis]] hyemalis'' and ''J. h. carolinensis'' |
||
|Photoperiodic cues drive earlier development of the gonads in ''J. h. carolinensis'' of whom migrate to a different region to breed while only residing sympatrically with ''J. h. hyemalis'' birds for half of the year.<ref>{{Citation|title=Reproductive allochrony in seasonally sympatric populations maintained by differential response to photoperiod: implications for population divergence and response to climate change |author=Fudickar AM, Greives TJ, Atwell JW, Stricker CA, and Ketterson ED |journal=American Naturalist |year=2016 |volume=187 |issue= |pages=436–446 |doi= |pmid= |url= }}</ref> |
|Photoperiodic cues drive earlier development of the gonads in ''J. h. carolinensis'' of whom migrate to a different region to breed while only residing sympatrically with ''J. h. hyemalis'' birds for half of the year.<ref>{{Citation|title=Reproductive allochrony in seasonally sympatric populations maintained by differential response to photoperiod: implications for population divergence and response to climate change |author=Fudickar AM, Greives TJ, Atwell JW, Stricker CA, and Ketterson ED |journal=American Naturalist |year=2016 |volume=187 |issue= 4|pages=436–446 |doi= 10.1086/685296|pmid= 27028072|s2cid=16006406 |url= }}</ref> |
||
|- |
|- |
||
|''[[Daphnia pulex]]'' and ''[[Daphnia pulicaria|D. pulicaria]]'' |
|''[[Daphnia pulex]]'' and ''[[Daphnia pulicaria|D. pulicaria]]'' |
||
|Very limited isolation is detected between the two water flea species (''D. pulicaria'' is within the ''D. pulex'' complex) possibly the result of reproductive timing based on photoperiodic cues.<ref>{{Citation|title=Photoperiodic response of sexual reproduction in the ''Daphnia pulex'' group is reversed in two distinct habitats |author=H-W. Deng |journal=Limnology and Oceanography |year=1997 |volume=42 |issue= |pages=609–611 |doi= |pmid= |url= }}</ref> |
|Very limited isolation is detected between the two water flea species (''D. pulicaria'' is within the ''D. pulex'' complex) possibly the result of reproductive timing based on photoperiodic cues.<ref>{{Citation|title=Photoperiodic response of sexual reproduction in the ''Daphnia pulex'' group is reversed in two distinct habitats |author=H-W. Deng |journal=Limnology and Oceanography |year=1997 |volume=42 |issue= 3|pages=609–611 |doi= 10.4319/lo.1997.42.3.0609|pmid= |url= }}</ref> |
||
|- |
|- |
||
|''[[Tibicina]]'' |
|''[[Tibicina]]'' |
||
|Habitat isolation, allochrony, and allopatry were identified in varioius pairs of seven species and subspecies of the genus (''T. Corsica Corsica'', ''T. Corsica fairmairei'', ''T. garricola'', ''T haematodes'', ''T. nigronervosa'', ''T. quadrisignata'', and ''T. tomentosa'').<ref>{{Citation|title=Spatial and ecological isolation in cicadas: first data from Tibicina (Hemiptera: Cicadoidea) in France |author=Jérôme Sueur and Stéphane Puissant |journal=European Journal of Entomology |year=2002 |volume=99 |issue= |pages= |doi=10.14411/EJE.2002.063 |pmid= |url=https://pdfs.semanticscholar.org/0631/eef1481b20f9f04af90ac5247650154eacfa.pdf?_ga=2.86273040.928958334.1608142471-1608880449.1608142471 }}</ref> |
|Habitat isolation, allochrony, and allopatry were identified in varioius pairs of seven species and subspecies of the genus (''T. Corsica Corsica'', ''T. Corsica fairmairei'', ''T. garricola'', ''T haematodes'', ''T. nigronervosa'', ''T. quadrisignata'', and ''T. tomentosa'').<ref>{{Citation|title=Spatial and ecological isolation in cicadas: first data from Tibicina (Hemiptera: Cicadoidea) in France |author=Jérôme Sueur and Stéphane Puissant |journal=European Journal of Entomology |year=2002 |volume=99 |issue= 4|pages= 477–484|doi=10.14411/EJE.2002.063 |pmid= |s2cid=56042738 |url=https://pdfs.semanticscholar.org/0631/eef1481b20f9f04af90ac5247650154eacfa.pdf?_ga=2.86273040.928958334.1608142471-1608880449.1608142471 }}</ref> |
||
|- |
|- |
||
|''[[Kaltenbachiella japonica]]'' |
|''[[Kaltenbachiella japonica]]'' |
||
|The galling aphids depend on the budding of [[Ulmus davidiana var. japonica|Japanese elm]] to hatch. Incipient populations have arisen due to changes in the budding times of the host plant.<ref>{{Citation|title=Genetic differentiation as a result of adaptation to the phenologies of individual host trees in the galling aphid ''Kaltenbachiella japonica'' |author=Komatsu T and Akimoto S |journal=Ecological Entomology |year=1995 |volume=20 |issue= |pages=33–42 |doi= |pmid= |url= }}</ref> |
|The galling aphids depend on the budding of [[Ulmus davidiana var. japonica|Japanese elm]] to hatch. Incipient populations have arisen due to changes in the budding times of the host plant.<ref>{{Citation|title=Genetic differentiation as a result of adaptation to the phenologies of individual host trees in the galling aphid ''Kaltenbachiella japonica'' |author=Komatsu T and Akimoto S |journal=Ecological Entomology |year=1995 |volume=20 |issue= |pages=33–42 |doi= 10.1111/j.1365-2311.1995.tb00426.x|pmid= |s2cid=83596331 |url= }}</ref> |
||
|- |
|- |
||
|''[[Scolioneura betuleti]]'' and ''[[Scolioneura vicina|S. vicina]]'' |
|''[[Scolioneura betuleti]]'' and ''[[Scolioneura vicina|S. vicina]]'' |
||
|The leaf-mining sawflies, despite being very similar, show some evidence of divergence due to seasonal flying (fall and spring respectively).<ref>{{Citation|title=Phylogenetics and evolution of host-plant use in leaf-mining sawflies (Hymenoptera: Tenthredinidae: Heterarthrinae) |author=Leppänen SA, Altenhofer E, Liston AD, and Nyman T |journal=Molecular Phylogenetics and Evolution |year=2012 |volume=64 |issue= |pages=331–341 |doi= |pmid= |url= }}</ref><ref>{{Citation|title=Mitochondrial DNA variation in two invasive birch leaf-mining sawflies in North America |author= MacQuarrie CJK, Langor DW, and Sperling FAH |journal=Canadian Entomology |year=2007 |volume=139 |issue= |pages=545–553 |doi= |pmid= |url= }}</ref> |
|The leaf-mining sawflies, despite being very similar, show some evidence of divergence due to seasonal flying (fall and spring respectively).<ref>{{Citation|title=Phylogenetics and evolution of host-plant use in leaf-mining sawflies (Hymenoptera: Tenthredinidae: Heterarthrinae) |author=Leppänen SA, Altenhofer E, Liston AD, and Nyman T |journal=Molecular Phylogenetics and Evolution |year=2012 |volume=64 |issue= 2|pages=331–341 |doi= 10.1016/j.ympev.2012.04.005|pmid= 22531610|url= }}</ref><ref>{{Citation|title=Mitochondrial DNA variation in two invasive birch leaf-mining sawflies in North America |author= MacQuarrie CJK, Langor DW, and Sperling FAH |journal=Canadian Entomology |year=2007 |volume=139 |issue= 4|pages=545–553 |doi= 10.4039/n06-084|pmid= |s2cid= 85614594 |url= }}</ref> |
||
|- |
|- |
||
|''[[Papilio canadensis]]'' and ''[[Papilio glaucus|P. glaucus]]'' |
|''[[Papilio canadensis]]'' and ''[[Papilio glaucus|P. glaucus]]'' |
||
|The two butterfly species have hybridized creating a hybrid population that breeds during a different time than the parent populations. Genetic evidence indicates that genes control the timing of reproduction.<ref>{{Citation|title=Impacts of climate warming on hybrid zone movement: geographically diffuse and biologically porous |
|The two butterfly species have hybridized creating a hybrid population that breeds during a different time than the parent populations. Genetic evidence indicates that genes control the timing of reproduction.<ref>{{Citation|title=Impacts of climate warming on hybrid zone movement: geographically diffuse and biologically porous 'species borders' |author=Scriber JM |journal=Insect Science |year=2011 |volume=18 |issue= 2|pages=121–159 |doi= 10.1111/j.1744-7917.2010.01367.x|pmid= |s2cid=86586378 |url= }}</ref><ref>{{Citation|title=Allochronic isolation and incipient hybrid speciation in tiger swallowtail butterflies |author=Ording GJ, Mercader RJ, Aardema ML, and Scriber JM |journal=Oecologia |year=2010 |volume=162 |issue= 2|pages=523–531 |doi= 10.1007/s00442-009-1493-8|pmid= 19937057|s2cid=22623508 |url= }}</ref> |
||
|- |
|- |
||
|''[[Tyrannus savana]]'' |
|''[[Tyrannus savana]]'' |
||
|Fork-tailed flycatcher populations are diverging due to isolation by a change in breeding times and breeding grounds as a result of a loss of migratory behavior.<ref>{{Citation|title=Speciation Associated with Shifts in Migratory Behavior in an Avian Radiation |author=Valentina Gómez-Bahamón, Roberto Márquez, Alex E.Jahn, Cristina Yumi Miyaki, Diego T. Tuero, Oscar Laverde-R, Silvia Restrepo, and Carlos Daniel Cadena |journal=Current Biology |year=2020 |volume=30 |issue=7 |pages= |
|Fork-tailed flycatcher populations are diverging due to isolation by a change in breeding times and breeding grounds as a result of a loss of migratory behavior.<ref>{{Citation|title=Speciation Associated with Shifts in Migratory Behavior in an Avian Radiation |author=Valentina Gómez-Bahamón, Roberto Márquez, Alex E.Jahn, Cristina Yumi Miyaki, Diego T. Tuero, Oscar Laverde-R, Silvia Restrepo, and Carlos Daniel Cadena |journal=Current Biology |year=2020 |volume=30 |issue=7 |pages=1312–1321 |doi=10.1016/j.cub.2020.01.064 |pmid= 32197080|s2cid=214585322 |url= }}</ref> |
||
|- |
|- |
||
|''[[European corn borer|Ostrinia nubilalis]]'' |
|''[[European corn borer|Ostrinia nubilalis]]'' |
||
|Corn borer moth strains breed at differing times of the night are considered to be incipient, however it is unclear if allochrony is exclusively causing isolation.<ref>{{Citation|title=Laboratory hybridization and mating period studies using two pheromone strains of ''Ostrinia nubilalis'' |author=Liebherr J. and Roelofs W. |journal=Annals of the Entomological Society of America |year=1975 |volume=68 |issue=2 |pages=305–309 |doi= 10.1093/aesa/68.2.305 }}</ref> Seasonal breeding may keep North American populations isolated as the number of yearly broods ([[voltinism]]) between the two strains. Sympatric to each other, Z strain is monovoltine (having a single brood in a year) and the E strain is divoltine (having two broods in a year).<ref name="Dopmanetal2010">{{Citation|title=Components of reproductive isolation between North American pheromone strains of the European corn borer |author=Dopman EB, Robbins PS, and Seaman A |journal=Evolution |year=2010 |volume=64 |issue= 4|pages=881–902 |doi=10.1111/j.1558-5646.2009.00883.x|pmid=19895559 |s2cid=9909878 }}</ref> In Europe, there is a correlation between larval emergence time and the host plant. <ref>{{Citation|title=Genetic isolation between two sympatric host-plant race of the European corn borer, ''Ostrina nubilalis'' Hübner. I. sex pheromone, moth emergence timing, and parasitism |author=Thomas Y, Bethenod M-T, Pelozuelo L, Frérot B, and Bourguet D |journal=Evolution |year=2003 |volume=57 |issue=2 |pages=261–273 |doi=10.1111/j.0014-3820.2003.tb00261.x |pmid=12683523 |s2cid=221734366 |url=https://hal.inrae.fr/hal-02671702/file/Thomas%20et%20al.%20Evolution%202003.pdf}}</ref> |
|Corn borer moth strains breed at differing times of the night are considered to be incipient, however it is unclear if allochrony is exclusively causing isolation.<ref>{{Citation|title=Laboratory hybridization and mating period studies using two pheromone strains of ''Ostrinia nubilalis'' |author=Liebherr J. and Roelofs W. |journal=Annals of the Entomological Society of America |year=1975 |volume=68 |issue=2 |pages=305–309 |doi= 10.1093/aesa/68.2.305 }}</ref> Seasonal breeding may keep North American populations isolated as the number of yearly broods ([[voltinism]]) between the two strains. Sympatric to each other, Z strain is monovoltine (having a single brood in a year) and the E strain is divoltine (having two broods in a year).<ref name="Dopmanetal2010">{{Citation|title=Components of reproductive isolation between North American pheromone strains of the European corn borer |author=Dopman EB, Robbins PS, and Seaman A |journal=Evolution |year=2010 |volume=64 |issue= 4|pages=881–902 |doi=10.1111/j.1558-5646.2009.00883.x|pmid=19895559 |pmc=2857697 |s2cid=9909878 }}</ref> In Europe, there is a correlation between larval emergence time and the host plant. <ref>{{Citation|title=Genetic isolation between two sympatric host-plant race of the European corn borer, ''Ostrina nubilalis'' Hübner. I. sex pheromone, moth emergence timing, and parasitism |author=Thomas Y, Bethenod M-T, Pelozuelo L, Frérot B, and Bourguet D |journal=Evolution |year=2003 |volume=57 |issue=2 |pages=261–273 |doi=10.1111/j.0014-3820.2003.tb00261.x |pmid=12683523 |s2cid=221734366 |url=https://hal.inrae.fr/hal-02671702/file/Thomas%20et%20al.%20Evolution%202003.pdf}}</ref> |
||
|- |
|- |
||
|New World bird species |
|New World bird species |
||
|In an experiment testing the [[Asynchrony of Seasons Hypothesis]], 57 different bird species found across the [[New World]] (North and South America and nearby landmasses) were found to express increased genetic differentiation in correlation with living in areas that have asynchronous [[precipitation]].<ref>{{Citation|title=Asynchrony of seasons: genetic differentiation associated with geographic variation in climatic seasonality and reproductive phenology |author=Ignacio Quintero, Sebastián González-Caro, Paul-Camilo Zalamea, and Carlos Daniel Cadena |journal=American Naturalist |year=2014 |volume=184 |issue=3 |pages= |
|In an experiment testing the [[Asynchrony of Seasons Hypothesis]], 57 different bird species found across the [[New World]] (North and South America and nearby landmasses) were found to express increased genetic differentiation in correlation with living in areas that have asynchronous [[precipitation]].<ref>{{Citation|title=Asynchrony of seasons: genetic differentiation associated with geographic variation in climatic seasonality and reproductive phenology |author=Ignacio Quintero, Sebastián González-Caro, Paul-Camilo Zalamea, and Carlos Daniel Cadena |journal=American Naturalist |year=2014 |volume=184 |issue=3 |pages=352–363 |doi=10.1086/677261 |pmid=25141144 |s2cid=39670263 |url= }}</ref> |
||
|} |
|} |
||
=== Asynchrony of Seasons Hypothesis === |
=== Asynchrony of Seasons Hypothesis === |
||
A noteworthy and significant pattern in nature is that of [[latitudinal gradients in species diversity]].<ref>{{Citation|title=Latitudinal Gradients of Biodiversity: Pattern, Process, Scale, and Synthesis |author=M.R. Willig, D.M. Kaufman, and R.D. Stevens |journal=Annual Review of Ecology, Evolution, and Systematics |year=2003 |volume=34 |issue= |pages= |
A noteworthy and significant pattern in nature is that of [[latitudinal gradients in species diversity]].<ref>{{Citation|title=Latitudinal Gradients of Biodiversity: Pattern, Process, Scale, and Synthesis |author=M.R. Willig, D.M. Kaufman, and R.D. Stevens |journal=Annual Review of Ecology, Evolution, and Systematics |year=2003 |volume=34 |issue= |pages=273–309 |doi=10.1146/annurev.ecolsys.34.012103.144032 |pmid= |url= }}</ref><ref name=pmid14970922>{{cite journal |last1=Hillebrand |first1=H |title=On the generality of the latitudinal diversity gradient. |journal=The American Naturalist |date=February 2004 |volume=163 |issue=2 |pages=192–211 |doi=10.1086/381004 |pmid=14970922 |s2cid=9886026 |url=http://oceanrep.geomar.de/4048/1/Hillebrand_2004_Amer_nat.pdf }}</ref> where species' richness increases closer to Earth's equator. It is thought that one contributing factor is that rates of speciation are higher in these regions across the planet.<ref>{{cite book |last1=Schemske |first1=Doug |editor-last1=Butlin |editor-first1=Roger K. |editor-last2=Bridle |editor-first2=Jon |editor-last3=Schluter |editor-first3= Dolph |title=Speciation and patterns of diversity |publisher=Oxford University Press |date=2009 |pages=219–239 |chapter=Biotic interactions and speciation in the tropics |isbn=9780511815683 |name-list-style=amp}}</ref> The [[Asynchrony of Seasons Hypothesis]] is proposed to be a contributing factor to higher speciation rates as it relates directly to that of allochronic speciation.<ref name="Martinetal2009">{{Citation|title=Latitudinal variation in the asynchrony of seasons: implications for higher rates of population differentiation and speciation in the tropics |author=Paul R. Martin, Frances Bonier, Ignacio T. Moore, and Joshua J. Tewksbury |journal=Ideas in Ecology and Evolution |year=2009 |volume=2 |issue= |pages=9–17 |doi=10.4033/iee.2009.2.3.n |pmid= |url= }}</ref> |
||
== References == |
== References == |
||
Revision as of 21:15, 19 December 2020
Allochronic speciation (also known as allochronic isolation, or temporal isolation is a form of speciation (specifically ecological speciation) arising from reproductive isolation that occurs due to a change in breeding time that reduces or eliminates gene flow between two populations of a species. The term allochrony is used to describe the general ecological phenomenon of the differences in phenology that arise between two or more species—speciation caused by allochrony is effectively allochronic speciation.
Environmental changes acting on a species population or populations can drive isolation. An important form of isolation is when populations are separated, not geographically, but temporally (by time). Genetic changes (mutations) over time can cause the two populations to differ—notably in phenology (events in a species life dictated by time such as breeding seasons); exhibiting unique phenotypes (the observable characteristics or traits of an organism).
Scientists have developed models to explain how this process occurs and how it is detected in natural populations. A wealth of studies exist regarding species in allochrony, with a select few that strongly suggest species are speciating or already have speciated as a direct consequence of this mode of isolation.
Model
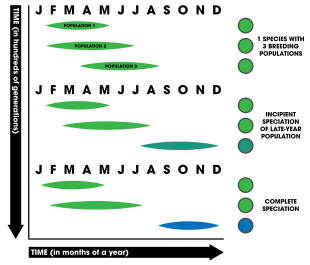
Speciation ultimately results due to the reproductive isolation between two populations. This can happen in a multitude of ways, a common mode of which is known as allopatric speciation. The geographic mode, where two species become physically isolated and unable to interbreed, allows for selection to act on both populations independently. Overtime, this gives rise to a new species.[1]: 86 Allochronic speciation is a form is isolation that can involve allopatry; however, it is not required.[1]: 208
Allochrony can involve a number of factors that induce the formation of a new species. Organisms have evolved various reproductive strategies (e.g. semelparity and iteroparity, single or multiple reproductive cycles in a lifetime) that can result in different outcomes for allochrony. Many organisms also breed at different times of the day, different seasons in the year, and even over multiple years or decades.[2] Seasonal breeding in animals is a common occurrence as well as spawning (in aquatic animals) times.[1]: 202 In plants, breeding in regards to time could involve the the receptivity of the stigma (the female part of the flower) to accepting sperm, periods of pollen release (such as in conifer trees where male cones disperse pollen relying on wind to direct pollen to female cones), or the overall timing of flowering (based on possible environmental cues such as moisture levels, soil type or quality, temperature, or photoperiod).[1]: 202–206 Even migratory patterns can play a role, as species may become isolated due to migrating at different times and to different locations.[3] Climate change is considered to have a significant impact on allochrony—in particular, seasonal breeding species.[2] Modeling changes in species breeding patterns due to climate as well as understanding the genetic mechanisms that control it has proven to important.[2]
Because of these many factors, slight to major changes in phenology can drive divergence between two populations. For example, a species with multiple breeding seasons in a year may shift those times depending on external conditions such as temperature or predation. In the event the populations (either allopatrically or sympatrically distributed, started breeding at different times, it would prevent members of each population from exchanging genes with one another. Over time, if genes are not exchanged, genetic differences arise in each population. If natural selection acts strongly on the two populations, they may become reproductively isolated, unable to reproduce viable, fertile offspring.
For allochronic speciation to be considered to have actually occurred, the model necessitates three major requirements:[2]
- Phylogenetic analysis must indicate that the two taxa in question are incipient species or clearly sister taxa.
- Breeding timing is required to be genetically-based (heritable) as opposed to changeable throughout life (phenotypic plasticity.
- The source of divergence can be determined to be explicitly allochrony and not the result of reinforcement or other evolutionary mechanisms.
Allochrony is thought to evolve more easily the greater the heritability of reproductive timing—that is, the greater the link between genes and the timing of reproduction—the more likely speciation will occur.[4] Allochrony can non-genetic;[1]: 203 however genetic factors must be involved for isolation to lead to complete reproductive isolation and subsequent speciation. The time frames involving allochrony are typically divided into three categories (prevalence in nature as well as examples are provided alongside each category):[2]
- Daily (considered to be common, examples include stony corals such as Acropora[5] or Orbicella[6].
- Seasonally (considered to be the most common, seasonal breeding times often coincide with winter, spring, fall, or summer; examples include salmon breeding runs such as in sockeye salmon.[7]
- Yearly (considered rarer, examples include periodical cicadas[8] and bamboo,[9] both of which reproduce within a scale of decades.
Population structures

A: Absent allochrony, only geographic and mate-choice cause isolation.
B: Starts with geographic separation, mate choice furthers isolation, and is completed by allochrony.
C: Starts with mate-choice differentiation followed by allochrony.
D: Mating and ecological factors accompany allochrony.
Other phenotypic traits are often found to co-occur with reproductive timing such as flowering number, egg-clutch sizes, reproductive lifespans, or body size—what can be defined as temporal phenotypic clines.[4] Two explanations exist for the existence these clines: phenotypic plasticity or phenotypic heritability (or possibly a combination of both). If plastic, the clines arise when certain phenotypic traits influence breeding time—such as reproducing at times when their traits are best suited or if conditions drive the expression of traits.[4] If heritable, the same factors may be expressed as they are in a plastic explanation; however, gene flow limitations allow for adaptation to the specific conditions of the reproductive time. This means that, "an individual with a heritable tendency to reproduce early that instead reproduced late might express traits typical of early reproducers".[4]
Isolation by time (IBT) is partially analogous to the concept of isolation by distance (IBD)[4] wherein genetic differences between populations increase with spatial distance.[11] When IBT is present in a population, the variation of natural selection during a breeding season causes adaptation by time (ABT) generating adaptive temporal variation in phenotypic traits. These two concepts are described in the following sections. Studies of salmonid fishes (involving reproductive lifespans, size at adulthood, age, energy storage, the mass of ovaries, egg sizes, number of eggs in a clutch, fecundity, and rates of development) and flowering plants (involving plant size, duration of flowing time, the number of flowers, the number of fruits, the timing of fruiting, and leaf size) have provided strong evidence of IBT leading to ABT[4] as well as studies of yearly allochrony.[2]
Isolation by time
The concept of IBT warrants two probabilities: in the event that heritability in reproductive timing exists among populations that breed during different seasons, probability of mating will be, "inversely proportional to the difference in the heritable component of their reproductive times."[4][12] The probability of mating can also be proportional to breeding values (phenotypic trait expressed as the trait of tis offspring) for reproductive time in the event the heritability is additive (more than one gene controls the phenotypic trait).[4] In a population, offspring will inherit the traits for reproductive time causing a decrease in gene flow while reproductive timing differences increase.[4]
Adaptation by time
Adaptation by time is an extension of divergence due to limited gene flow between populations experiencing different selective pressures.[13] Typically this is limited to spatial variation such as in ecological speciation; however, in allochrony, selection varies not just in space, but in reproductive time—giving rise to adaptive temporal clines in phenotypic traits that are heritable. Isolation by time effectively allows adaptive temporal clines to evolve as long as the reproductive season has selective variation. Evidence for adaptation by time demands four factors: 1) time restricts gene flow, 2) the reproductive season expresses variations in phenotypic traits, 3) temporal variation is controlled genetically (it is not plastic), and 4) temporal variation is adaptive.[4] ABT increases, "as (i) selection on the trait increases; (ii) environmental influences on reproductive time decrease; (iii) the heritability of reproductive time increases; and (iv) the temporal distribution of reproductive activity becomes increasingly uniform."[4]
Detecting allochrony

Because allochronic speciation can occur in conjunction with other modes and forms of speciation, researchers must attempt to determine if the initial stages of isolation were the result of allochrony. The speciation continuum of allopatry, parapatry, and sympatry have all been implicated in studies of temporal isolation.[1]: 206 Allochrony can also facilitate reinforcement after secondary contact.[2] The frequency of allochronic speciation is thought to common but understudied as allochrony is widespread in nature.[14]
Testing whether or not allochrony prevents gene flow can be difficult due to the multitude of unknown variables in wild populations and the inability to replicate and manipulate it in laboratory settings.[1]: 203 Producing viable, and fertile offspring (or the lack thereof) is not always possible; fortunately, lake of mate tests do not necessarily indicate temporal isolation is not at play.[1]: 203 As stated prior, one of the necessary criteria is that the species in question must be sister taxa (or demonstrably incipient). This means that accurate phylogenies are vital to determining the initial stage of a speciation event.[1]: 203
Despite the multitude of studies, it is not always evident whether allochrony is the sole driver of speciation or if other factors acting simultaneously are responsible.[2] This can be more challenging when speciation has already occurred (in that the taxa in question are reproductively isolated and no longer incipient).[2] Determining how important allochrony is as a historical cause of speciation can be tested by: 1) comparative studies that show the young pairs of sister taxa are temporally isolated and 2) testing cases of incipient species in sympatry where reproductive isolation is incomplete without temporal isolation.[1]: 206
Determining if allochrony is the source of divergence require a key pattern to be measured: isolation (and subsequently speciation) should correlate with a decrease in overlapping breeding times.[2] This pattern indicates that daily allochrony is more prone to gene flow (closeness of breeding times can allow accidental intermixing of populations) while yearly allochrony is the least prone to gene flow (accidental intermixing is rare if large time frames exist between mating periods).[2]
Examples of divergence driven by allochrony
The following table documents cases of allochronic speciation. Varying degrees of certainty exist as not all cases strongly meet the three primary criteria necessitated by allochronic speciation. Species marked with an asterisk (*) indicate stronger confidence assessed by Rebecca Taylor and Vicki Friesen (2017).[2]
| Species | Description |
|---|---|
| Acropora samoensis* and other Acropora spp.* | Japanese corals found to be reproductively isolated by the timing of their spawning.[5] Sympatric species populations of A. samoensis coral spawn separately in the fall and spring with spawning being a heritable, likely involving the PaxC gene.[15] |
| Montastraea annularis*, M. faveolata*, and M. franksi* | Three related species of coral that have speciated due to the timing of their spawning.[6] |
| Oncorhynchus nerka*, O. gorbuscha*, and O. tshawytscha | Yearly breeding runs of Sockeye salmon occur during two periods in the year (late and early) have caused genetic isolation of incipient populations. Salmon breeding is known to be genetic but no specific genes are known for this species.[7][16][17] Even and odd two-year life cycles in conjunction with seasonal breeding runs of pink salmon (O. gorbuscha) has driven genetic differentiation between the two populations.[18][19][20][21][22] Breeding run times also vary across the population range of the Chinook salmon (O. tshawytscha).[23][24] |
| Thaumetopoea pityocampa* | Codominance in genes is associated with the emergence time for larval stages of this moth species. Winter and summer larval populations are in the process of speciating.[25][26][27] |
| Inurois punctigera* | Breeding is prevented in areas where mid-winter temperatures are unsuitable for the moth species. This has given rise to late and early populations.[28] |
| Pemphigus populi-transversus* and P. obesinymphae* | The gall-forming aphids produce galls on different leaves of the same host tree species. P. populi-transversus forms galls on early spring leaves while P. obesinymphae forms them on leaves in the summer. This has lead to full reproductive isolation.[29] |
| Asphondylia spp.* | Three midge species infect the stems of Larrea tridentata, A. auripila in summer, A. resinosa in winter, and A. foliosa in spring.[30] |
| Cellana spp.* | Inhabiting different depths within centimeters, the limpets have become reproductively isolated likely due to a combination of parapatric speciation and spawn cues (e.g. spawning according to water level.[31] |
| Hydrobates spp.* | The petrels group has reproductively isolated (in the Azores) and incipient species (other archipelagos) caused by cool and warm breeding seasons.[32][33][34] |
| Howea belmoreana* and H. forsteriana* | Genetically controlled flowering times have caused (in conjunction with differing soil pH levels) the reproductive isolation of two palm species on Lord Howe Island.[35] |
| Erysiphe necator* | Exhibits evidence of isolation due to temporal differences of its host species Vitis vinifera.[36] |
| Magicicada spp.* | Groups of 13- and 17-year life cycle species pairs (seven species total) of cicada emerge to reproduce separated by large time frames between breading seasons.[37][38][8] Only every 221 years do the 13 and 17 year cycles align where both pairs emerge simultaneously.[2] |
| Antitrogus parvulus* | Two beetle cohorts express genetic differentiation from life cycles separated by two-year intervals.[39] |
| Oeneis melissa semidea* | Two-year life cycles of the butterfly species breeding groups have caused genetic differentiation.[40] |
| Bambusoideae* | Bamboo under go semelparous reproduction where they live for years before mass-flowering at once. This can happen in different years and different locations. Allochronic patches are thought to have driven the diversification of global bamboo species.[41][9][42] |
| Spodoptera frugiperda | A phytophagous example of two moth larvae strains breeding on either corn or rice at different times of the night. Other causes of isolation may be acting on the species. The population in the United States appears to be speciating via allochrony;[43][44] however the population in Columbia does not.[45] |
| Anopheles gambiae and A. coluzzii | Controlled by circadian rhythms that stimulate mating, the mosquitos swarm at slightly different times during twilight exhibit some evifence of allochrony, though it is possible that reinforcement or microallopatric speciation is at play.[46][47] |
| Bactrocera tryoni and B. neohumeralis | Only laboratory hybridization has been observed between the two Queensland fruit flies, the latter of which mates only during the day time, while the former mates only at night.[48] |
| Anastrepha bistrigata and A. striata | The fruit flies mate during during morning and afternoon respectively. Courtship behavior could also be isolating the two species.[49] |
| Salmo salar | The age at full maturation as well as genetic differentiation varies between one- and three-year Atlantic salmon (these are the years in which the young fish leave to the ocean and return to their breeding grounds).[50] |
| Anguilla anguilla | European eels have varying rates that they mature based on environmental factors. This creates separated breeding populations that show some genetic differentiation—notably between 2-3 year breeding intervals.[51] |
| Cuculus canorus | Allochrony likely plays a role in the Cuckoo bird as they depend on host species for rearing their young. Hosts lay eggs at different times, and cuckoos depend on these timeframes to replace a host bird's eggs.[52] |
| Antechinus spp. | The marsupial mice respond strongly to photoperiodic cues and in sympatric populations, reproductively isolated species are found to breed at different times.[53] |
| Coregonus clupeaformis | The lake whitefish has two known forms, normal and dwarf. They have different spawning times but may have diverged in allopatry.[54][55] |
| Exapion ulicis and E. lemovicinum | E. lemovicinum infects Ulex minor and U. gallii plants while E. ulicis infects U. europaeus. The timing in which they lay eggs on the plant occurs in fall and spring respectively.[56] |
| Meconopsis autumnalis and M. paniculata | Himalayan poppy are a fully reproductively isolated species thought to have speciated through allochrony as they exist in sympatry and flower at different times in the season.[57] |
| Cordia spp. | Some of the species in the genus exhibit significant variation in flowering times.[58] |
| Hesperiidae | It is thought that temporal isolation is responsible for speciation in many of the 400 skipper butterfly species studied.[59] |
| Bryopsidales spp. | The green algae reproduces by releasing gametes at different times. This is thought to have driven reproductive isolation, but it is unclear if it is genetically controlled or based purely on environmental cues.[60] |
| Chilo suppressalis | Mate timing occurs at different intervals at night as well as dependence on different host plants.[61] |
| Prodoxus quinquepunctellus | Host races of the moths inhabit Adam’s needle and thread yucca with larval emergence occurring in conjunction with flowering time. It is thought that morphology and host-shifting contribute alongside allochrony.[62] |
| Gryllus pennsylvanicus and G. veletis | The spring field crickets have been described as speciating in allochrony due to their maturation timing.[63] However more recent studies indicate that they are not sister species.[64] |
| Haemaphysalis spp. and Dermacentor spp. | Three Hungarian tick species in the Haemaphysalis genus exhibit mating activity in sympatry during three seasonal periods, late fall, late spring, and early spring. Two tick species in the Dermacentor genus show peak activity in fall and spring.[65] |
| Strauzia longipennis | Genetic variation is detected in three sunflower maggot fly variants that inhabit the same host plant. Their larval emergence occurs in three distinct periods of the summer keeping them partially isolated. Experimental manipulation suggests allochrony will increase as they continue to diverge.[66][67] |
| Enchenopa binotata complex | The phenology of members in the treehopper species complex is correlated with their host plants—when changing host species in experiment, the treehopper egg hatching time changes promoting assortive mating.[68] |
| Milicia excelsa | With flowering time of the African teak thought to be genetically controlled, the population exhibits isolation.[69] |
| Asteralobia sasakii | Two populations of Cecidomyiidae gall midges differ substantially in emergence time (with no overlap) on two different Ilex hosts. [70] |
| Salix spp. | The Canadian willow species are found to isolated by flowering time; three of which flower early (S. bebbiana, S. discolor, S. eriocephala, and S. petiolaris) and late (S. amygdaloides, S. exigua, and S. lucida). Hybrids are not known outside of laboratory settings and exhibit intermediate flowering times. All seven species exist in sympatric distributions.[71] |
| Juncus effusus | Sympatric populations of genetically differentiated plants flower at different times preventing hybridization. It is unclear if speciation is occurring by allochrony as reinforcement may be a stronger explanation.[72] |
| Agrostis tenuis | The grass species A. tenuis grows on soil contaminated with high levels of copper leeched from an unused mine. Adjacent is the non-contaminated soil. The populations are evolving reproductive isolation due to differences in flowering time.[73] |
| Anthoxanthum odoratum | The grass species A. odoratum grows on soil contaminated with high levels of lead and zinc leeched from an unused mine. Adjacent is the non-contaminated soil. The populations are evolving reproductive isolation due to differences in flowering time.[73] |
| Chironomus nuditarsis | The non-biting midge (genus Chironomus) exhibits differences in life cycle in accordance with elevation.[74] |
| Terellia fuscicornis | Differences in courtship behavior as well as morphology are found in populations that infect different hosts (Silybum marianumand Cynara) that bloom at different times.[75] |
| Ampelomyces spp. | Genetically different strains of the mycoparasitic fungus that infects apple powdery mildew completes their lifecycle before other strains that infect other mildew hosts.[76] |
| Glycine max and G. soja | Wild soybean (G. soja) and cultivated soybean (G. max) can be prevented from hybridizing by inducing asynchrony in flowering time.[77] This study is unique in that it is not an example of allochronic speciation, but instead an experiment demonstrating that allochrony can be experimentally applied to induce isolation. |
| Parasitoid wasps: Rhagoletis pomonella, Rhagoletis mendax, Diachasma alloeum, Diachasmimorpha mellea, and Utetes canaliculatus | In R. pomonella (one of the most researched, model organisms), genetic data indicates heritability of emergence and its associated flight time.[78] In commercial blueberry fields versus wild ones, the populations of R. mendax differ in their flight periods causing a reduction in gene flow.[79] Other Rhagoletis species that host on Crataegus show similar patterns.[80] Cospeciation of the parasitoid wasps (D. alloeum, D. mellea, and U. canaliculatus) and their host plant apple maggot has been induced by host-shifts caused by various factors such as timing of its egg hatching, fruit smell preference, philopatry, and avoidance. The egg hatching timing factor implicates allochrony.[81] |
| Eurosta solidaginis | Two populations of goldenrod gall fly differ in their emergence periods on their host plants Solidago altissima and S. gigantea by 10 to 14 days preventing hybridization and causing isolation.[82] |
| Falco sparverius | Kestrels of Idaho have both migrating and year-round residents with the year-round population nesting earlier generating assortive mating.[83] |
| Sylvia atricapilla | Some genetic differentiation exists between blackcap populations that migrate to the United Kingdom and Ireland versus those that migrate to Iberia. The birds breed in sympatry in Germany; however, the UK and Ireland populations migrate back earlier causing assortive mating. Hybrids end up with intermediate migration routes.[84] |
| Junco hyemalis hyemalis and J. h. carolinensis | Photoperiodic cues drive earlier development of the gonads in J. h. carolinensis of whom migrate to a different region to breed while only residing sympatrically with J. h. hyemalis birds for half of the year.[85] |
| Daphnia pulex and D. pulicaria | Very limited isolation is detected between the two water flea species (D. pulicaria is within the D. pulex complex) possibly the result of reproductive timing based on photoperiodic cues.[86] |
| Tibicina | Habitat isolation, allochrony, and allopatry were identified in varioius pairs of seven species and subspecies of the genus (T. Corsica Corsica, T. Corsica fairmairei, T. garricola, T haematodes, T. nigronervosa, T. quadrisignata, and T. tomentosa).[87] |
| Kaltenbachiella japonica | The galling aphids depend on the budding of Japanese elm to hatch. Incipient populations have arisen due to changes in the budding times of the host plant.[88] |
| Scolioneura betuleti and S. vicina | The leaf-mining sawflies, despite being very similar, show some evidence of divergence due to seasonal flying (fall and spring respectively).[89][90] |
| Papilio canadensis and P. glaucus | The two butterfly species have hybridized creating a hybrid population that breeds during a different time than the parent populations. Genetic evidence indicates that genes control the timing of reproduction.[91][92] |
| Tyrannus savana | Fork-tailed flycatcher populations are diverging due to isolation by a change in breeding times and breeding grounds as a result of a loss of migratory behavior.[93] |
| Ostrinia nubilalis | Corn borer moth strains breed at differing times of the night are considered to be incipient, however it is unclear if allochrony is exclusively causing isolation.[94] Seasonal breeding may keep North American populations isolated as the number of yearly broods (voltinism) between the two strains. Sympatric to each other, Z strain is monovoltine (having a single brood in a year) and the E strain is divoltine (having two broods in a year).[14] In Europe, there is a correlation between larval emergence time and the host plant. [95] |
| New World bird species | In an experiment testing the Asynchrony of Seasons Hypothesis, 57 different bird species found across the New World (North and South America and nearby landmasses) were found to express increased genetic differentiation in correlation with living in areas that have asynchronous precipitation.[96] |
Asynchrony of Seasons Hypothesis
A noteworthy and significant pattern in nature is that of latitudinal gradients in species diversity.[97][98] where species' richness increases closer to Earth's equator. It is thought that one contributing factor is that rates of speciation are higher in these regions across the planet.[99] The Asynchrony of Seasons Hypothesis is proposed to be a contributing factor to higher speciation rates as it relates directly to that of allochronic speciation.[100]
References
- ^ a b c d e f g h i j Jerry A. Coyne; H. Allen Orr (2004), Speciation, Sinauer Associates, pp. 1–545, ISBN 0-87893-091-4
- ^ a b c d e f g h i j k l m n o Rebecca S. Taylor and Vicki L. Friesen (2017), "The role of allochrony in speciation", Molecular Ecology, 26 (13): 3330–3342, doi:10.1111/mec.14126, PMID 28370658, S2CID 46852358
- ^ Sheela P. Turbek, Elizabeth S.C. Scordato, and Rebecca J. Safran (2018), "The Role of Seasonal Migration in Population Divergence and Reproductive Isolation", Trends in Ecology & Evolution, 33 (3): 164–175, doi:10.1016/j.tree.2017.11.008, PMID 29289354
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l Andrew P. Hendry and Troy Day (2005), "Population structure attributable to reproductive time: isolation by time and adaptation by time", Molecular Ecology, 14 (4): 901–916, doi:10.1111/j.1365-294X.2005.02480.x, PMID 15773924, S2CID 8226535
- ^ a b H. Fukami, M. Omori, K. Shimoike, T. Hayashibara, and M. Hatta (2003), "Ecological and genetic aspects of reproductive isolation by different spawning times in Acropora corals", Marine Biology, 142 (4): 679–684, doi:10.1007/s00227-002-1001-8, S2CID 81981786
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b N. Knowlton, J. L. Maté, H. M. Guzmán, R. Rowan, and J. Jara (1997), "Direct evidence for reproductive isolation among the three species of the Montastraea annularis complex in Central America (Panamá and Honduras)", Marine Biology, 127 (4): 705–711, doi:10.1007/s002270050061, S2CID 37997956
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Andrew P. Hendry, Ole K. Berg, and Thomas P. Quinn (1999), "Condition dependence and adaptation-by-time: breeding date, life history, and energy allocation within a population of salmon", Oikos, 85 (3): 499–514, doi:10.2307/3546699, JSTOR 3546699
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Teiji Sota, Satoshi Yamamoto, John R. Cooley, Kathy B. R. Hill, Chris Simon, and Jin Yoshimura (2013), "Independent divergence of 13- and 17-y life cycles among three periodical cicada lineages", PNAS, 110 (17): 6919–6924, Bibcode:2013PNAS..110.6919S, doi:10.1073/pnas.1220060110, PMC 3637745, PMID 23509294
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Donald C. Franklin (2004), "Synchrony and asynchrony: observations and hypotheses for the flowering wave in a long‐lived semelparous bamboo", Journal of Biogeography, 31 (5): 773–786, doi:10.1111/j.1365-2699.2003.01057.x
- ^ Dieckmann U., Doebeli M., Metz JAJ, & Tautz D. (2004), Adaptive Speciation (PDF), Cambridge, UK: Cambridge University Press, doi:10.2277/0521828422 (inactive 2020-12-15), ISBN 9781107404182
{{citation}}: CS1 maint: DOI inactive as of December 2020 (link) CS1 maint: multiple names: authors list (link) - ^ Montgomery Slatkin (1993). "Isolation by Distance in Equilibrium and Non-Equilibrium Populations". Evolution. 47 (1): 264–279. doi:10.2307/2410134. JSTOR 2410134. PMID 28568097.
- ^ Gordon A. Fox (2003), "Assortative mating and plant phenology: evolutionary and practical consequences" (PDF), Evolutionary Ecology Research, 5: 1–18
- ^ Dolph Schluter (2000), The Ecology of Adaptive Radiation, Oxford University Press, ISBN 0198505221
- ^ a b Dopman EB, Robbins PS, and Seaman A (2010), "Components of reproductive isolation between North American pheromone strains of the European corn borer", Evolution, 64 (4): 881–902, doi:10.1111/j.1558-5646.2009.00883.x, PMC 2857697, PMID 19895559, S2CID 9909878
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Natalie L Rosser (2015), "Asynchronous spawning in sympatric populations of a hard coral reveals cryptic species and ancient genetic lineages", Molecular Ecology, 24 (19): 5006–5019, doi:10.1111/mec.13372, PMID 26339867, S2CID 13151100
- ^ Andrew P Hendry, Yolanda E Morbey, Ole K Berg, and John K Wenburg (2004), "Adaptive variation in senescence: reproductive lifespan in a wild salmon population", Proceedings of the Royal Society B: Biological Sciences, 271 (1536): 259–266, doi:10.1098/rspb.2003.2600, PMC 1691593, PMID 15058436
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ E. K. Fillatre, P. Etherton, and D. D. Heath (2003), "Bimodal run distribution in a northern population of sockeye salmon (Oncorhynchus nerka): life history and genetic analysis on a temporal scale", Molecular Ecology, 12 (7): 1793–1805, doi:10.1046/j.1365-294x.2003.01869.x, PMID 12803632, S2CID 25772120
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Lev A. Zhivotovsky, A. J. Gharrett, A. J. McGregor, M. K. Glubokovsky, and Marcus W. Feldman (1994), "Gene differentiation in Pacific salmon (Oncorhynchus Sp.): facts and models with reference to pink salmon (O. Gorbuscha)", Canadian Journal of Fisheries and Aquatic Sciences, 51: 223–232, doi:10.1139/f94-308
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ D. Churikov and A. J. Gharrett (2002), "Comparative phylogeography of the two pink salmon broodlines: an analysis based on a mitochondrial DNA genealogy", Molecular Ecology, 11 (6): 1077–1101, doi:10.1046/j.1365-294x.2002.01506.x, PMID 12030984, S2CID 24965183
- ^ Morten T. Limborg, Ryan K. Waples, James E. Seeb, and Lisa W. Seeb (2014), "Temporally Isolated Lineages of Pink Salmon Reveal Unique Signatures of Selection on Distinct Pools of Standing Genetic Variation", Journal of Heredity, 105 (6): 835–845, doi:10.1093/jhered/esu063, PMID 25292170
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Smoker WW, Gharrett AJ, and Stekoll MS (1998), "Genetic variation of return date in a population of pink salmon: a consequence of fluctuating environment and dispersive selection?", Alaska Fishery Research Bulletin, 5: 46–54
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Gharrett AJ, Lane S, McGregor AJ, and Taylor SG (2001), "Use of a genetic marker to examine genetic interaction among subpopulations of pink salmon (Oncorhynchus gorbuscha)", Genetica, 111 (1–3): 259–267, doi:10.1023/A:1013791314900, PMID 11841171, S2CID 19278664
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Quinn TP, Unwin MJ, and Kinnison MT (2000), "Evolution of temporal isolation in the wild: genetic divergence in timing of migration and breeding by introduced chinook salmon populations", Evolution, 54 (4): 1372–1385, doi:10.1111/j.0014-3820.2000.tb00569.x, PMID 11005303, S2CID 23316021
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Brieuc MSO, Ono K, Drinan DP, and Naish KA (2015), "Integration of Random Forest with population-based outlier analyses provides insight on the genomic basis and evolution of run timing in Chinook salmon (Oncorhynchus tshawytscha)", Molecular Ecology, 24 (11): 2729–2746, doi:10.1111/mec.13211, PMID 25913096, S2CID 206182207
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ C.Pimentel, T.Calvão, M.Santos, C.Ferreira, M.Neves, and J.-Å.Nilsson (2006), "Establishment and expansion of a Thaumetopoea pityocampa (Den. & Schiff.) (Lep. Notodontidae) population with a shifted life cycle in a production pine forest, Central-Coastal Portugal", Forest Ecology and Management, 233 (1): 108–115, doi:10.1016/j.foreco.2006.06.005
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Helena M Santos, Maria-Rosa Paiva, Susana Rocha, Carole Kerdelhué, and Manuela Branco (2013), "Phenotypic divergence in reproductive traits of a moth population experiencing a phenological shift", Ecology and Evolution, 3 (15): 5098–5108, doi:10.1002/ece3.865, PMC 3892371, PMID 24455139
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Manuela Branco, Maria-Rosa Paiva, Helena Maria Santos, Christian Burban, and Carole Kerdelhué (2017), "Experimental evidence for heritable reproductive time in 2 allochronic populations of pine processionary moth", Insect Science, 24 (2): 325–335, doi:10.1111/1744-7917.12287, PMID 26530538, S2CID 9091980
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Satoshi Yamamoto and Teiji Sota (2012), "Parallel allochronic divergence in a winter moth due to disruption of reproductive period by winter harshness", Molecular Ecology, 21 (1): 174–183, doi:10.1111/j.1365-294X.2011.05371.x, PMID 22098106, S2CID 23572464
- ^ Patrick Abbot and James H Withgott (2004), "Phylogenetic and molecular evidence for allochronic speciation in gall-forming aphids (Pemphigus)", Evolution, 58 (3): 539–553, doi:10.1111/j.0014-3820.2004.tb01677.x, PMID 15119438, S2CID 25277034
- ^ Jeffrey B Joy and Bernard J Crespi (2007), "Adaptive radiation of gall-inducing insects within a single host-plant species", Evolution, 61 (4): 784–795, doi:10.1111/j.1558-5646.2007.00069.x, PMID 17439611, S2CID 16864372
- ^ Christopher E. Bird, Brenden S. Holland, Brian W Bowen, and Robert J Toonen (2011), "Diversification of sympatric broadcast-spawning limpets (Cellana spp.) within the Hawaiian archipelago", Molecular Ecology, 20 (10): 2128–2141, doi:10.1111/j.1365-294X.2011.05081.x, PMID 21481050, S2CID 23432529
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ L. R. Monteiro (1998), "Speciation through temporal segregation of Madeiran storm petrel (Oceanodroma castro) populations in the Azores?", Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 353 (1371): 945–953, doi:10.1098/rstb.1998.0259, PMC 1692297
- ^ V. L. Friesen, A. L. Smith, E. Gómez-Díaz, M. Bolton, R. W. Furness, J. González-Solís, and L. R. Monteiro (2007), "Sympatric speciation by allochrony in a seabird" (PDF), PNAS, 104 (47): 18589–18594, Bibcode:2007PNAS..10418589F, doi:10.1073/pnas.0700446104, PMC 2141821, PMID 18006662
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Mark Bolton, Andrea L. Smith, Elena Gómez‐díaz, Vicki L. Friesen, Renata Medeiros, Joël Bried, Jose L. Roscales, and Robert W. Furness (2008), "Monteiro's Storm‐petrel Oceanodroma monteiroi: a new species from the Azores", Ibis, 150 (4): 717–727, doi:10.1111/j.1474-919X.2008.00854.x
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Vincent Savolainen, Marie-Charlotte Anstett, Christian Lexer, Ian Hutton, James J Clarkson, Maria V Norup, Martyn P Powell, David Springate, Nicolas Salamin, and William J Baker (2006), "Sympatric speciation in palms on an oceanic island", Nature, 441 (7090): 210–213, Bibcode:2006Natur.441..210S, doi:10.1038/nature04566, PMID 16467788, S2CID 867216
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Josselin Montarry, Philippe Cartolaro, Sylvie Richard-Cervera, and François Delmotte (2009), "Spatio-temporal distribution of Erysiphe necator genetic groups and their relationship with disease levels in vineyards", European Journal of Plant Pathology, 123: 61–70, doi:10.1007/s10658-008-9343-9, S2CID 13114251
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ D. C. Marshall and J. R. Cooley (2000), "Reproductive character displacement and speciation in periodical cicadas, with description of new species, 13-year Magicicada neotredecem", Evolution, 54 (4): 1313–1325, doi:10.1111/j.0014-3820.2000.tb00564.x, PMID 11005298, S2CID 28276015
- ^ C. Simon, J. Tang, S. Dalwadi, G. Staley, J. Deniega, and T. R. Unnasch (2000), "Genetic evidence for assortative mating between 13-year cicadas and sympatric "17-year cicadas with 13-year life cycles" provides support for allochronic speciation", Evolution, 54 (4): 1326–1336, doi:10.1111/j.0014-3820.2000.tb00565.x, PMID 11005299, S2CID 19105047
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ D. P. Logan, P. G. Allsopp, and M. P. Zalucki (2003), "Overwintering, soil distribution and phenology of Childers canegrub, Antitrogus parvulus (Coleoptera: Scarabaeidae) in Queensland sugarcane", Bulletin of Entomological Research, 93 (4): 307–314, doi:10.1079/ber2003245, PMID 12908916
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ A. E. Gradish, N. Keyghobadi, and G. W. Otis (2015), "Population genetic structure and genetic diversity of the threatened White Mountain arctic butterfly (Oeneis melissa semidea)", Conservation Genetics, 16 (5): 1253–1264, doi:10.1007/s10592-015-0736-y, S2CID 13307002
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Madhav Gadgil and S. Narendra Prasad (1984), "Ecological Determinants of Life History Evolution of Two Indian Bamboo Species", Biotropica, 16 (3): 161–172, doi:10.2307/2388050, JSTOR 2388050
- ^ Anelena L. de Carvalho, Bruce W. Nelson, Milton C. Bianchini, Daniela Plagnol, Tatiana M. Kuplich, and Douglas C. Daly (2013), "Bamboo-Dominated Forests of the Southwest Amazon: Detection, Spatial Extent, Life Cycle Length and Flowering Waves", PLOS ONE, 8 (1): e54852, Bibcode:2013PLoSO...854852C, doi:10.1371/journal.pone.0054852, PMC 3554598, PMID 23359438
{{citation}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Schöfl G, Dill A, Heckel DG, and Groot AT (2011), "Allochronic separation versus mate choice: nonrandom patterns of mating between fall armyworm host strains", American Naturalist, 177 (4): 470–485, doi:10.1086/658904, PMID 21460569, S2CID 12528391
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Schöfl G, Heckel DG, and Groot AT (2009), "Time-shifted reproductive behaviours among fall armyworm (Noctuidae: Spodoptera frugiperda) host strains: Evidence for differing modes of inheritance", Journal of Evolutionary Biology, 22 (7): 1447–1459, doi:10.1111/j.1420-9101.2009.01759.x, PMID 19467132, S2CID 22004781
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Saldamando-Benjumea CI, Estrada-Piedrahíta K, Velásquez-Vélez MI, and Bailey RI (2014), "Assortative mating and lack of temporality between corn and rice strains of Spodoptera frugiperda (Lepidoptera, Noctuidae) from central Colombia", Journal of Insect Behaviour, 27 (5): 555–566, doi:10.1007/s10905-014-9451-7, S2CID 16310075
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Samuel S C Rund, Samuel J Lee, Brian R Bush, and Giles E Duffield (2012), "Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes", Journal of Insect Physiology, 58 (12): 1609–1619, doi:10.1016/j.jinsphys.2012.09.016, PMID 23068991
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Simon P Sawadogo, Carlo Costantini, Cédric Pennetier, Abdoulaye Diabaté, Gabriella Gibson, and Roch K Dabiré (2013), "Differences in timing of mating swarms in sympatric populations of Anopheles coluzzii and Anopheles gambiae s.s. (formerly An. gambiae M and S molecular forms) in Burkina Faso, West Africa", Parasites & Vectors, 6 (275): 275–288, doi:10.1186/1756-3305-6-275, PMC 3851435, PMID 24330578
{{citation}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ N. Pike, W. Y. S. Wang, and A. Meats (2003), "The likely fate of hybrids of Bactrocera tryoni and Bactrocera neohumeralis", Heredity, 90 (5): 365–370, doi:10.1038/sj.hdy.6800253, PMID 12714981, S2CID 34631721
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Denise Selivon and João S. Morgante (1997), "Reproductive isolation between Anastrepha bistrigata and A. striata (Diptera, Tephritidae)", Brazilian Journal of Genetics, 20 (4): 583–585, doi:10.1590/S0100-84551997000400005
- ^ Susan E Johnston, Panu Orell, Victoria L Pritchard, Matthew P Kent, Sigbjørn Lien, Eero Niemelä, Jaakko Erkinaro, and Craig R Primmer (2014), "Genome-wide SNP analysis reveals a genetic basis for sea-age variation in a wild population of Atlantic salmon (Salmo salar)", Molecular Ecology, 23 (14): 3452–3468, doi:10.1111/mec.12832, PMID 24931807, S2CID 11737380
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Maes GE, Pujolar JM, Hellemans B, and Volckaert FAM (2006), "Evidence for isolation by time in the European eel (Anguilla anguilla L.)", Molecular Ecology, 15 (8): 2095–2107, doi:10.1111/j.1365-294X.2006.02925.x, PMID 16780427, S2CID 10586483
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ A P Møller, A Antonov, B G Stokke, F Fossøy, A Moksnes, E Røskaft, and F Takasu (2011), "Isolation by time and habitat and coexistence of distinct host races of the common cuckoo", Journal of Evolutionary Biology, 24 (3): 676–684, doi:10.1111/j.1420-9101.2010.02202.x, PMID 21214656, S2CID 24056512
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ McAllan BM, Dickman CR, and Crowther MS (2006), "Photoperiod as a reproductive cue in the marsupial genus Antechinus: Ecological and evolutionary consequences", Biological Journal of the Linnean Society, 87 (3): 365–379, doi:10.1111/j.1095-8312.2006.00571.x
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Kirkpatrick M, and Selander RK (1979), "Genetics of speciation in lake whitefishes in the Allegash Basin", Evolution, 33 (1Part2): 478–485, doi:10.1111/j.1558-5646.1979.tb04700.x, PMID 28568177, S2CID 32131280
- ^ Rogers SM, Isabel N, and Bernatchez L (2007), "Linkage maps of the dwarf and normal lake whitefish (Coregonus clupeaformis) species complex and their hybrids reveal the genetic architecture of population divergence", Genetics, 175 (1): 375–398, doi:10.1534/genetics.106.061457, PMC 1774998, PMID 17110497
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Barat M, Tarayre M, and Atlan A (2007), "Plant phenology and seed predation: Interactions between gorses and weevils in Brittany (France)", Entomologia Experimentalis et Applicata, 124 (2): 167–176, doi:10.1111/j.1570-7458.2007.00565.x, S2CID 85880513
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Egan PA (2011), "Meconopsis autumnalis and M. manasluensis (Papaveraceae), two new species of Himalayan poppy endemic to central Nepal with sympatric congeners", Phytotaxa, 20: 47–56, doi:10.11646/phytotaxa.20.1.4
- ^ Opler PA, Baker HG, and Frankie GW (1975), "Reproductive biology of some Costa Rican Cordia species (Boraginaceae)", Biotropica, 7: 234–247, doi:10.2307/2989736, JSTOR 2989736
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Devries PJ, Austin GT, and Martin NH (2008), "Diel activity and reproductive isolation in a diverse assemblage of Neotropical skippers (Lepidoptera: Hesperiidae)", Biological Journal of the Linnean Society, 94 (4): 723–736, doi:10.1111/j.1095-8312.2008.01037.x
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Clifton K and Clifton L (1999), "The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs", Journal of Phycology, 35: 24–34, doi:10.1046/j.1529-8817.1999.3510024.x, S2CID 83704320
- ^ Ueno H, Furukawa S, and Tsuchida K (2006), "Difference in the time of mating activity between host-associated populations of the rice stem borer, Chilo suppressalis (Walker)", Entomological Science, 9 (3): 255–259, doi:10.1111/j.1479-8298.2006.00171.x, S2CID 86106927
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Groman JD and Pellmyr O (2000), "Rapid evolution and specialization following host colonization in a yucca moth", Journal of Evolutionary Biology, 13 (2): 223–236, doi:10.1046/j.1420-9101.2000.00159.x, S2CID 84556390
- ^ Alexander RD and Bigelow RS (1960), "Allochronic speciation in field crickets, and a new species, Acheta veletis", Evolution, 14 (3): 334–346, doi:10.1111/j.1558-5646.1960.tb03095.x, hdl:2027.42/137466, S2CID 87867996
- ^ Harrison RG, Bogdanowicz SM, and Hall C (1995), "Mitochondrial DNA phylogeny of North American field crickets: perspectives on the evolution of life cycles, songs, and habitat associations", Journal of Evolutionary Biology, 8 (2): 209–232, doi:10.1046/j.1420-9101.1995.8020209.x, S2CID 85777010
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Hornok, S. (2009), "Allochronic seasonal peak activities of Dermacentor and Haemaphysalis spp. under continental climate in Hungary", Veterinary Parasitology, 163 (4): 366–369, doi:10.1016/j.vetpar.2009.03.048, PMID 19410373
- ^ Hippee AC, Elnes ME, Armenta JS, Condon MA, and Forbes AA (2016), "Divergence before the host shift? Prezygotic reproductive isolation among three varieties of a specialist fly on a single host plant", Ecological Entomology, 41 (4): 389–399, doi:10.1111/een.12309, S2CID 87320676
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Forbes AA, Kelly PH, Middleton KA, and Condon MA (2013), "Genetically differentiated races and speciation-with-gene-flow in the sunflower maggot, Strauzia longipennis", Evolutionary Ecology, 27 (5): 1017–1032, doi:10.1007/s10682-012-9622-y, S2CID 18472327
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Wood TK, Olmstead KL, and Guttman SI (1990), "Insect phenology mediated by host-plant water relations", Evolution, 44 (3): 629–636, doi:10.1111/j.1558-5646.1990.tb05943.x, PMID 28567982, S2CID 43689173
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Kasso Daïnou, Eric Laurenty, Grégory Mahy, Olivier J Hardy, Yves Brostaux, Nikki Tagg, and Jean-Louis Doucet (2012), "Phenological patterns in a natural population of a tropical timber tree species, Milicia excelsa (Moraceae): Evidence of isolation by time and its interaction with feeding strategies of dispersers", American Journal of Botany, 99 (9): 1453–1463, doi:10.3732/ajb.1200147, PMID 22912370
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Tabuchi K, Amano H (2003), "Host-associated differences in emergence pattern, reproductive behavior and life history of Asteralobia sasakii (Monzen)(Diptera: Cecidomyiidae) between populations on Ilex crenata and I. integra (Aquifoliaceae)", Applied Entomology and Zoology, 38: 501–508, doi:10.1303/aez.2003.501
- ^ A. Mosseler and C. S. Papadopol (1989), "Seasonal isolation as a reproductive barrier among sympatric Salix species", Canadian Journal of Botany, 67 (9): 2563–2570, doi:10.1139/b89-331
- ^ Stefan G Michalski and Walter Durka (2015), "Separation in flowering time contributes to the maintenance of sympatric cryptic plant lineages", Ecology and Evolution, 5 (11): 2172–2184, doi:10.1002/ece3.1481, PMC 4461419, PMID 26078854
- ^ a b Thomas McNeilly and Janis Antonovics (1968), "Evolution in Closely Adjacent Plant Populations. IV. Barriers to Gene Flow", Heredity, 23 (2): 205–218, doi:10.1038/hdy.1968.29
- ^ Polukonova NV and Karmokov MK (2013), "Microevolutionary changes in populations of Chironomus nuditarsis Str. (Keyl, 1962) (Chironomidae, Diptera) from central Caucasus", Russian Journal of Genetics, 49 (2): 175–181, doi:10.1134/S1022795413020099, S2CID 12200060
- ^ Sayar NP, Smith CA, White IM, Knio KM (2009), "Terellia fuscicornis (Diptera: Tephritidae): biological and morphological adaptation on artichoke and milk thistle", Journal of Natural History, 43 (19–20): 1159–1181, doi:10.1080/00222930902807742, S2CID 56004378
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Levente Kiss, Alexandra Pintye, Gábor M Kovács, Tünde Jankovics, Michael C Fontaine, Nick Harvey, Xiangming Xu, Philippe C Nicot, Marc Bardin, Jacqui A Shykoff, and Tatiana Giraud (2011), "Temporal isolation explains host-related genetic differentiation in a group of widespread mycoparasitic fungi" (PDF), Molecular Ecology, 20 (7): 1492–1507, doi:10.1111/j.1365-294X.2011.05007.x, PMID 21261766, S2CID 34557058
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Ohigashi K, Mizuguti A, Yoshimura Y, Matsuo K, and Miwa T (2014), "A new method for evaluating flowering synchrony to support the temporal isolation of genetically modified crops from their wild relatives", Journal of Plant Research, 127 (1): 109–117, doi:10.1007/s10265-013-0592-0, PMID 24122370, S2CID 13606001
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Scott P Egan, Gregory J Ragland, Lauren Assour, Thomas H Q Powell, Glen R Hood, Scott Emrich, Patrik Nosil, and Jeffrey L Feder (2015), "Experimental evidence of genome-wide impact of ecological selection during early stages of speciation-with-gene-flow", Ecology Letters, 18 (8): 817–825, doi:10.1111/ele.12460, PMC 4744793, PMID 26077935
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Teixeira LAF and Polavarapu S (2003), "Evolution of phenologically distinct populations of Rhagoletis mendax (Diptera: Tephritidae) in Highbush Blueberry Fields", Annals of the Entomological Society of America, 96 (6): 818–827, doi:10.1603/0013-8746(2003)096[0818:EOPDPO]2.0.CO;2
- ^ Thomas H Q Powell, Andrew A Forbes, Glen R Hood, and Jeffrey L Feder (2014), "Ecological adaptation and reproductive isolation in sympatry: Genetic and phenotypic evidence for native host races of Rhagoletis pomonella", Molecular Ecology, 23 (3): 688–704, doi:10.1111/mec.12635, PMID 24351094, S2CID 2745741
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Glen R Hood, Andrew A Forbes, Thomas H Q Powell, Scott P Egan, Gabriela Hamerlinck, James J Smith, and Jeffrey L Feder (2015), "Sequential divergence and the multiplicative origin of community diversity", Proceedings of the National Academy of Sciences, 112 (44): E5980–E5989, doi:10.1073/pnas.1424717112, PMC 4640724, PMID 26499247
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Craig TP, Horner JD, and Itami JK (2001), "Genetics, experience, and host-plant preference in Eurosta solidaginis: implications for host shifts and speciation", Evolution, 55 (4): 773–782, doi:10.1554/0014-3820(2001)055[0773:GEAHPP]2.0.CO;2, PMID 11392395
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Anderson AM, Novak SJ, Smith JF, Steenhof K, Heath JA (2016), "Nesting phenology, mate choice, and genetic divergence within a partially migratory population of American Kestrels", The Auk, 133: 99–109, doi:10.1642/AUK-15-129.1, S2CID 85683278
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Claudia Hermes, Raeann Mettler, Diego Santiago-Alarcon, Gernot Segelbacher, and H. Martin Schaefer (2015), "Spatial Isolation and Temporal Variation in Fitness and Condition Facilitate Divergence in a Migratory Divide", PLOS ONE, 10 (12): e0144264, Bibcode:2015PLoSO..1044264H, doi:10.1371/journal.pone.0144264, PMC 4681481, PMID 26656955
{{citation}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Fudickar AM, Greives TJ, Atwell JW, Stricker CA, and Ketterson ED (2016), "Reproductive allochrony in seasonally sympatric populations maintained by differential response to photoperiod: implications for population divergence and response to climate change", American Naturalist, 187 (4): 436–446, doi:10.1086/685296, PMID 27028072, S2CID 16006406
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ H-W. Deng (1997), "Photoperiodic response of sexual reproduction in the Daphnia pulex group is reversed in two distinct habitats", Limnology and Oceanography, 42 (3): 609–611, doi:10.4319/lo.1997.42.3.0609
- ^ Jérôme Sueur and Stéphane Puissant (2002), "Spatial and ecological isolation in cicadas: first data from Tibicina (Hemiptera: Cicadoidea) in France" (PDF), European Journal of Entomology, 99 (4): 477–484, doi:10.14411/EJE.2002.063, S2CID 56042738
- ^ Komatsu T and Akimoto S (1995), "Genetic differentiation as a result of adaptation to the phenologies of individual host trees in the galling aphid Kaltenbachiella japonica", Ecological Entomology, 20: 33–42, doi:10.1111/j.1365-2311.1995.tb00426.x, S2CID 83596331
- ^ Leppänen SA, Altenhofer E, Liston AD, and Nyman T (2012), "Phylogenetics and evolution of host-plant use in leaf-mining sawflies (Hymenoptera: Tenthredinidae: Heterarthrinae)", Molecular Phylogenetics and Evolution, 64 (2): 331–341, doi:10.1016/j.ympev.2012.04.005, PMID 22531610
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ MacQuarrie CJK, Langor DW, and Sperling FAH (2007), "Mitochondrial DNA variation in two invasive birch leaf-mining sawflies in North America", Canadian Entomology, 139 (4): 545–553, doi:10.4039/n06-084, S2CID 85614594
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Scriber JM (2011), "Impacts of climate warming on hybrid zone movement: geographically diffuse and biologically porous 'species borders'", Insect Science, 18 (2): 121–159, doi:10.1111/j.1744-7917.2010.01367.x, S2CID 86586378
- ^ Ording GJ, Mercader RJ, Aardema ML, and Scriber JM (2010), "Allochronic isolation and incipient hybrid speciation in tiger swallowtail butterflies", Oecologia, 162 (2): 523–531, doi:10.1007/s00442-009-1493-8, PMID 19937057, S2CID 22623508
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Valentina Gómez-Bahamón, Roberto Márquez, Alex E.Jahn, Cristina Yumi Miyaki, Diego T. Tuero, Oscar Laverde-R, Silvia Restrepo, and Carlos Daniel Cadena (2020), "Speciation Associated with Shifts in Migratory Behavior in an Avian Radiation", Current Biology, 30 (7): 1312–1321, doi:10.1016/j.cub.2020.01.064, PMID 32197080, S2CID 214585322
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Liebherr J. and Roelofs W. (1975), "Laboratory hybridization and mating period studies using two pheromone strains of Ostrinia nubilalis", Annals of the Entomological Society of America, 68 (2): 305–309, doi:10.1093/aesa/68.2.305
- ^ Thomas Y, Bethenod M-T, Pelozuelo L, Frérot B, and Bourguet D (2003), "Genetic isolation between two sympatric host-plant race of the European corn borer, Ostrina nubilalis Hübner. I. sex pheromone, moth emergence timing, and parasitism" (PDF), Evolution, 57 (2): 261–273, doi:10.1111/j.0014-3820.2003.tb00261.x, PMID 12683523, S2CID 221734366
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Ignacio Quintero, Sebastián González-Caro, Paul-Camilo Zalamea, and Carlos Daniel Cadena (2014), "Asynchrony of seasons: genetic differentiation associated with geographic variation in climatic seasonality and reproductive phenology", American Naturalist, 184 (3): 352–363, doi:10.1086/677261, PMID 25141144, S2CID 39670263
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ M.R. Willig, D.M. Kaufman, and R.D. Stevens (2003), "Latitudinal Gradients of Biodiversity: Pattern, Process, Scale, and Synthesis", Annual Review of Ecology, Evolution, and Systematics, 34: 273–309, doi:10.1146/annurev.ecolsys.34.012103.144032
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Hillebrand, H (February 2004). "On the generality of the latitudinal diversity gradient" (PDF). The American Naturalist. 163 (2): 192–211. doi:10.1086/381004. PMID 14970922. S2CID 9886026.
- ^ Schemske, Doug (2009). "Biotic interactions and speciation in the tropics". In Butlin, Roger K.; Bridle, Jon & Schluter, Dolph (eds.). Speciation and patterns of diversity. Oxford University Press. pp. 219–239. ISBN 9780511815683.
- ^ Paul R. Martin, Frances Bonier, Ignacio T. Moore, and Joshua J. Tewksbury (2009), "Latitudinal variation in the asynchrony of seasons: implications for higher rates of population differentiation and speciation in the tropics", Ideas in Ecology and Evolution, 2: 9–17, doi:10.4033/iee.2009.2.3.n
{{citation}}: CS1 maint: multiple names: authors list (link)

