Carbon dioxide
This article may have too many links. (July, 2007) |
| Carbon dioxide | |||||
|---|---|---|---|---|---|
 
| |||||
| Other names | Carbonic acid gas,
Carbonic anhydride, dry ice (solid) | ||||
| Molecular formula | CO 2 | ||||
| Molar mass | 44.0095(14) g/mol | ||||
| Solid state | Dry ice, carbonia | ||||
| Appearance | colorless gas | ||||
| CAS number | [124-38-9] | ||||
| colspan="2" | | |||||
| Properties | |||||
| Density and phase | 1,600 kg/m³, solid
1.98 kg/m³, gas | ||||
| Solubility in water | 1.45 kg/m³ | ||||
| Latent heat of sublimation |
25.13 kJ/mol | ||||
| Melting point | −57 °C (216 K) (under pressure) | ||||
| Boiling point | −78 °C (195 K), (sublimes) | ||||
| Triple point | 518 kPa at −56.6°C | ||||
| Critical point | 7,821 kPa at 31.1°C | ||||
| Acidity (pKa) | 6.35 and 10.33 | ||||
| Viscosity | 0.07 cP at −78 °C | ||||
| Structure V | |||||
| Molecular shape | linear | ||||
| Crystal structure | quartz-like | ||||
| Dipole moment | zero | ||||
| Hazards | |||||
| MSDS | External MSDS | ||||
| Main hazards | asphyxiant, irritant | ||||
| NFPA 704 |
| ||||
| R-phrases | R: As, Fb | ||||
| S-phrases | Template:S9, Template:S23, Template:S36(liquid) | ||||
| RTECS number | FF6400000 | ||||
| Supplementary data page | |||||
| Structure & properties | n, εr, etc. | ||||
| Spectral data | UV, IR, NMR, MS | ||||
| Related compounds | |||||
| Related oxides | carbon monoxide carbon suboxide dicarbon monoxide carbon trioxide | ||||
| Except where noted otherwise, data are given for
materials in their standard state (at 25 °C, 100 kPa) | |||||
Carbon dioxide is a chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom. It is a gas at standard temperature and pressure and exists in Earth's atmosphere as a gas. It is currently at a globally averaged concentration of approximately 385 ppm by volume in the Earth's atmosphere, although this varies both by location and time. Carbon dioxide's chemical formula is CO
2.
In general, it is exhaled by animals and utilized by plants during photosynthesis. Additional carbon dioxide is created by the combustion of fossil fuels or vegetable matter, among other chemical processes.
Carbon dioxide is an important greenhouse gas because of its ability to absorb many infrared wavelengths of the Sun's light, and because of the length of time it stays in the Earth's atmosphere. Due to this, and the role it plays in the respiration of plants, it is a major component of the carbon cycle.
In its solid state, carbon dioxide is commonly called dry ice. Carbon dioxide has no liquid state at pressures below 5.1 atm.
Chemical and physical properties

Carbon dioxide is a colorless, odorless gas. When inhaled at concentrations higher than usual atmospheric levels, it can produce a sour taste in the mouth and a stinging sensation in the nose and throat. These effects result from the gas dissolving in the mucous membranes and saliva, forming a weak solution of carbonic acid. This sensation can also occur during an attempt to stifle a burp after drinking a carbonated beverage. Amounts above 800 ppm are considered unhealthy, amounts above 5,000 ppm are considered very unhealthy, and those above about 50,000 ppm are considered dangerous to animal life.[1]
At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m³, about 1.5 times that of air. The carbon dioxide molecule (O=C=O) contains two double bonds and has a linear shape. It has no electrical dipole, and as it is fully oxidized, it is not very reactive and is non-flammable.

At −78.5° C, carbon dioxide changes directly from a solid phase to a gaseous phase through sublimation, or from gaseous to solid through deposition. Solid carbon dioxide is normally called "dry ice", a generic trademark. It was first observed in 1825 by the French chemist Charles Thilorier. Dry ice is commonly used as a versatile cooling agent, and it is relatively inexpensive. As it warms, solid carbon dioxide sublimes directly into the gas phase, making its use convenient as it leaves no liquid. It can often be found in groceries and laboratories, and it is also used in the shipping industry. The largest non-cooling use for dry ice is blast cleaning.
Liquid carbon dioxide forms only at pressures above 5.1 atm; the triple point of carbon dioxide is about 518 kPa at −56.6°C (See phase diagram, above). The critical point is 7,821 kPa at 31.1°C.
An alternative form of solid carbon dioxide, an amorphous glass-like form, is possible, although not at atmospheric pressure.[2] This form of glass, called carbonia, was produced by supercooling heated CO
2 at extreme pressure (40–48 GPa or about 400,000 atmospheres) in a diamond anvil. This discovery confirmed the theory that carbon dioxide could exist in a glass state similar to other members of its elemental family, like silicon (silica glass) and germanium. Unlike silica and germania glasses, however, carbonia glass is not stable at normal pressures and reverts back to gas when pressure is released.
History of human understanding
Carbon dioxide was one of the first gases to be described as a substance distinct from air. In the seventeenth century, the Flemish chemist Jan Baptist van Helmont observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a "gas" or "wild spirit" (spiritus sylvestre).
The properties of carbon dioxide were studied more thoroughly in the 1750s by the Scottish physician Joseph Black. He found that limestone (calcium carbonate) could be heated or treated with acids to yield a gas he called "fixed air." He observed that the fixed air was denser than air and did not support either flame or animal life. He also found that when bubbled through an aqueous solution of lime (calcium hydroxide), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, English chemist Joseph Priestley published a paper entitled Impregnating Water with Fixed Air in which he described a process of dripping sulfuric acid (or oil of vitriol as Priestley knew it) on chalk in order to produce carbon dioxide, and forcing the gas to dissolve by agitating a bowl of water in contact with the gas.[3]
Carbon dioxide was first liquefied (at elevated pressures) in 1823 by Humphry Davy and Michael Faraday.[4] The earliest description of solid carbon dioxide was given by Charles Thilorier, who in 1834 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a "snow" of solid CO
2.[5]
Isolation
Carbon dioxide may be obtained from air distillation. However, this yields only very small quantities of CO
2. A large variety of chemical reactions yield carbon dioxide, such as the reaction between most acids and most metal carbonates. For example, the reaction between sulfuric acid and calcium carbonate (limestone or chalk) is depicted below:
- H
2SO
4+ CaCO
3→ CaSO
4+ H
2CO
3
The H
2CO
3 then decomposes to water and CO
2. Such reactions are accompanied by foaming or bubbling, or both. In industry such reactions are widespread because they can be used to neutralize waste acid streams.
The production of quicklime (CaO) a chemical that has widespread use, from limestone by heating at about 850 °C also produces CO
2:
- CaCO
3→ CaO + CO
2
The combustion of all carbon containing fuels, such as methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), but also of coal and wood, will yield carbon dioxide and, in most cases, water. As an example the chemical reaction between methane and oxygen is given below.
- CH
4+ 2 O
2→ CO
2+ 2 H
2O
Iron is reduced from its oxides with coke in a blast furnace, producing pig iron and carbon dioxide:
- 2 Fe
2O
3+ 3 C → 4 Fe + 3 CO
2
Yeast produces carbon dioxide and ethanol, also known as alcohol, in the production of wines, beers and other spirits:
- C
6H
12O
6 → 2 CO
2+ 2 C
2H
5OH
All aerobic organisms produce CO
2 when they oxidize carbohydrates, fatty acids, and proteins in the mitochondria of cells. CO
2 is the prime energy source and the main metabolic pathway in heterotroph organisms such as animals, and also a secondary energy source in phototroph organisms such as plants when not enough light is available for photosynthesis. The large number of reactions involved are exceedingly complex and not described easily. Refer to (cellular respiration, anaerobic respiration and photosynthesis). Photoautotrophs (i.e. plants, cyanobacteria) use another modus operandi: They absorb the CO
2 from the air, and, together with water, react it to form carbohydrates:
- nCO
2+ nH
2O → (CH
2O)
n+ nO
2
Carbon dioxide is soluble in water, in which it spontaneously interconverts between CO
2 and H
2CO
3 (carbonic acid). The relative concentrations of CO
2, H
2CO
3, and the deprotonated forms HCO-
3 (bicarbonate) and CO2-
3(carbonate) depend on the pH. In neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater, while in very alkaline water (pH > 10.4) the predominant (>50%) form is carbonate. The bicarbonate and carbonate forms are very soluble, such that air-equilibrated ocean water (mildly alkaline with typical pH = 8.2 – 8.5) contains about 120 mg of bicarbonate per liter.
Industrial production
Carbon dioxide is manufactured mainly from six processes:[6]
- As a byproduct in ammonia and hydrogen plants, where methane is converted to CO
2; - From combustion of carbonaceous fuels;
- As a byproduct of fermentation;
- From thermal decomposition of CaCO
3; - As a byproduct of sodium phosphate manufacture;
- Directly from natural carbon dioxide gas wells.
Uses
Carbon dioxide is used by the food industry, the oil industry, and the chemical industry.[6] It is used in many consumer products that require pressurized gas, because is inexpensive, nonflammable, and it undergoes a phase transition from gas to liquid at room temperature at an attainable pressure of approximately 60 bar, allowing far more carbon dioxide to fit in a given container than otherwise would. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminum capsules are also sold as supplies of compressed gas for airguns, paintball markers, for inflating bicycle tires, and for making seltzer. Rapid vaporization of liquid CO
2 is used for blasting in coal mines.
Carbon dioxide is used to produce carbonated soft drinks and soda water. Traditionally, the carbonation in beer and sparkling wine comes about through natural fermentation, but some manufacturers carbonate these drinks artificially. A candy called Pop Rocks is pressurized with carbon dioxide gas at about 40 bar (600 psi). When placed in the mouth, it dissolves (just like other hard candy) and releases the gas bubbles with an audible "pop."
Leavening agents produce carbon dioxide to cause dough to rise. Baker's yeast produces carbon dioxide by fermentation within the dough, while chemical leaveners such as baking powder and baking soda release carbon dioxide when heated or if exposed to acids.

Carbon dioxide is the most commonly used compressed gas for pneumatic systems in combat robots.
Carbon dioxide extinguishes flames, and some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide also finds use as an atmosphere for welding, although in the welding arc, it reacts to oxidize most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are brittler than those made in more inert atmospheres, and that such weld joints deteriorate over time because of the formation of carbonic acid. It is used as a welding gas primarily because it is much less expensive than more inert gases such as argon or helium.
Liquid carbon dioxide is a good solvent for many organic compounds, and is used to remove caffeine from coffee. First, the green coffee beans are soaked in water. The beans are placed in the top of a column seventy feet (21 meters) high. The carbon dioxide fluid at about 93 degrees Celsius enters at the bottom of the column. The caffeine diffuses out of the beans and into the carbon dioxide.
Carbon dioxide has begun to attract attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It's used by some dry cleaners for this reason. (See green chemistry.)
Plants require carbon dioxide to conduct photosynthesis, and greenhouses may enrich their atmospheres with additional CO
2 to boost plant growth. It has been proposed that carbon dioxide from power generation be bubbled into ponds to grow algae that could then be converted into biodiesel fuel[1]. High levels of carbon dioxide in the atmosphere effectively exterminate many pests. Greenhouses will raise the level of CO
2 to 10,000 ppm (1%) for several hours to eliminate pests such as whiteflies, spider mites, and others.
In medicine, up to 5% carbon dioxide is added to pure oxygen for stimulation of breathing after apnea and to stabilize the O
2/CO
2 balance in blood.
A common type of industrial gas laser is the carbon dioxide laser.
Carbon dioxide can also be combined with limonene oxide from orange peels or other epoxides to create polymers and plastics[7].
Carbon dioxide is commonly injected into or adjacent to producing oil wells. It acts as both a pressurizing agent and, when dissolved into the underground crude oil, significantly reduces its viscosity, enabling the oil to flow more rapidly through the earth to the removal well. In mature oil fields, extensive pipe networks are used to carry the carbon dioxide to the injection points.
In the chemical industry, carbon dioxide is used for the production of urea, carbonates and bicarbonates, and sodium salicylate.
Liquid and solid carbon dioxide are important refrigerants, especially in the food industry, where they are employed during the transportation and storage of ice cream and other frozen foods. Solid carbon dioxide is called "dry ice" and is used for small shipments where refrigeration equipment is not practical.
Liquid carbon dioxide (industry nomenclature R744 / R-744) was used as a refrigerant prior to the discovery of R-12 and is likely to enjoy a renaissance [8] due to environmental concerns. Its physical properties are highly favorable for cooling, refrigeration, and heating purposes, having a high volumetric cooling capacity. Due to its operation at pressures of up to 130 bars, CO
2 systems require highly resistant components that have been already developed to serial production in many sectors. In car air conditioning, in more than 90% of all driving conditions, R744 operates more efficient than systems using R-134a. Its environmental advantages (GWP of 1, non-ozone depleting, non-toxic, non-flammable) could make it the future working fluid to replace current HFCs in cars, supermarkets, hot water heat pumps, among others. Some applications: Coca-Cola has fielded CO
2-based beverage coolers[9] and the US Army[10] is interested in CO2 Technology.
By end-2007, the global car industry is expected to decide on the next-generation refrigerant in car air conditioning. CO
2 is one discussed option.(see The Cool War)
In the Earth's atmosphere
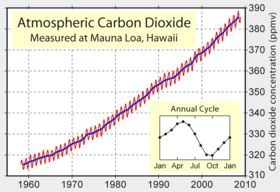
2 concentrations measured at Mauna Loa Observatory.
Carbon dioxide in earth's atmosphere is considered a trace gas, and is measured in parts per million. Current concentration levels average approximately 385 ppm, which represents a total of around 800 gigatons of carbon. Its concentration can vary considerably on a regional basis: in urban areas it is generally higher, and indoors can reach 10 times the atmospheric concentration.
Carbon dioxide is a greenhouse gas; see greenhouse effect for more.
Due to human activities such as the combustion of fossil fuels and deforestation, the concentration of atmospheric carbon dioxide has increased by about 35% since the beginning of the age of industrialization.
Up to 40% of the gas emitted by a volcano during a subaerial volcanic eruption is carbon dioxide.[11] However, human activities currently release more than 130 times the amount of CO
2 emitted by volcanoes. According to the best estimates, volcanoes release about 130-230 million tonnes (145-255 million tons) of CO
2 into the atmosphere each year. Emissions of CO
2 by human activities amount to about 27 billion tonnes per year (30 billion tons)[12].
Biological role
Carbon dioxide is an end product in organisms that obtain energy from breaking down sugars, fats and amino acids with oxygen as part of their metabolism, in a process known as cellular respiration. This includes all plants, animals, many fungi and some bacteria. In higher animals, the carbon dioxide travels in the blood from the body's tissues to the lungs where it is exhaled. In plants using photosynthesis, carbon dioxide is absorbed from the atmosphere.
Role in photosynthesis
Plants remove carbon dioxide from the atmosphere by photosynthesis, also called carbon assimilation, which uses light energy to produce organic plant materials by combining carbon dioxide and water. Free oxygen is released as gas from the decomposition of water molecules, while the hydrogen is split into its protons and electrons and used to generate chemical energy via photophosphorylation. This energy is required for the fixation of carbon dioxide in the Calvin cycle to form sugars. These sugars can then be used for growth within the plant through respiration. Carbon dioxide gas must be introduced into greenhouses to maintain plant growth, as even in vented greenhouses the concentration of carbon dioxide can fall during daylight hours to as low as 200 ppm, at which level photosynthesis is significantly reduced. Venting can help offset the drop in carbon dioxide, but will never raise it back to ambient levels of 340 ppm. Carbon dioxide supplementation is the only known method to overcome this deficiency. Direct introduction of pure carbon dioxide is ideal, but rarely done because of cost constraints. Most greenhouses burn methane or propane to supply the additional CO
2, but care must be taken to have a clean burning system as increased levels of nitrogen oxides (NO
x) result in reduced plant growth. Sensors for sulfur dioxide (SO
2) and NO
x are expensive and difficult to maintain; accordingly most systems come with a carbon monoxide (CO) sensor under the assumption that high levels of carbon monoxide mean that significant amounts of NO
x are being produced. Plants can potentially grow up to 50 percent faster in concentrations of 1,000 ppm CO
2 when compared with ambient conditions.[13]
Plants also emit CO
2 during respiration, so it is only during growth stages that plants are net absorbers. For example a growing forest will absorb many tonnes of CO
2 each year, however a mature forest will produce as much CO
2 from respiration and decomposition of dead specimens (e.g. fallen branches) as used in biosynthesis in growing plants.[citation needed] Regardless of this, mature forests are still valuable carbon sinks, helping maintain balance in the Earth's atmosphere. Additionally, and crucially to life on earth, phytoplankton photosynthesis absorbs dissolved CO
2 in the upper ocean and thereby promotes the absorption of CO
2 from the atmosphere.[14]
Animal toxicity
Carbon dioxide content in fresh air varies between 0.03% (300 ppm) and 0.06% (600 ppm), depending on the location (see graphical map of CO
2 in real-time). A person's exhaled breath is approximately 4.5% carbon dioxide. It is dangerous when inhaled in high concentrations (greater than 5% by volume, or 50,000 ppm). The current threshold limit value (TLV) or maximum level that is considered safe for healthy adults for an eight-hour work day is 0.5% (5,000 ppm). The maximum safe level for infants, children, the elderly and individuals with cardio-pulmonary health issues is significantly less.
These figures are valid for pure carbon dioxide. In indoor spaces occupied by people the carbon dioxide concentration will reach higher levels than in pure outdoor air. Concentrations higher than 1,000 ppm will cause discomfort in more than 20% of occupants, and the discomfort will increase with increasing CO
2 concentration. The discomfort will be caused by various gases coming from human respiration and perspiration, and not by CO
2 itself. At 2,000 ppm the majority of occupants will feel a significant degree of discomfort, and many will develop nausea and headaches. The CO
2 concentration between 300 and 2,500 ppm is used as an indicator of indoor air quality.
Acute carbon dioxide toxicity is sometimes known as by the names given to it by miners: black damp, choke damp, or stythe. Miners would try to alert themselves to dangerous levels of carbon dioxide in a mine shaft by bringing a caged canary with them as they worked. The canary would inevitably die before CO
2 reached levels toxic to people. Choke damp caused a great loss of life at Lake Nyos in Cameroon in 1986, when an upwelling of CO
2-laden lake water quickly blanketed a large surrounding populated area. The heavier carbon dioxide forced out the life-sustaining oxygen near the surface, killing nearly two thousand people.
Carbon dioxide ppm levels (CDPL) are a surrogate for measuring indoor pollutants that may cause occupants to grow drowsy, get headaches, or function at lower activity levels. To eliminate most Indoor Air Quality complaints, total indoor CDPL must be reduced to below 600. NIOSH considers that indoor air concentrations that exceed 1,000 are a marker suggesting inadequate ventilation. ASHRAE recommends they not exceed 1,000 inside a space. OSHA limits concentrations in the workplace to 5,000 for prolonged periods. The U.S. National Institute for Occupational Safety and Health limits brief exposures (up to ten minutes) to 30,000 and considers CDPL exceeding 40,000 as "immediately dangerous to life and health." People who breathe 50,000 for more than half an hour show signs of acute hypercapnia, while breathing 70,000 – 100,000 can produce unconsciousness in only a few minutes. Accordingly, carbon dioxide, either as a gas or as dry ice, should be handled only in well-ventilated areas.
Human physiology
CO
2 is carried in blood in three different ways. (The exact percentages vary depending whether it is arterial or venous blood).
- Most of it (about 80% – 90%) is converted to bicarbonate ions HCO3− by the enzyme carbonic anhydrase in the red blood cells.[15]
- 5% – 10% is bound to hemoglobin as carbamino compounds[15]
The CO
2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the N-terminal groups on the four globin chains. However, because of allosteric effects on the hemoglobin molecule, the binding of CO
2 decreases the amount of oxygen that is bound for a given partial pressure of oxygen.
Hemoglobin, the main oxygen-carrying molecule in red blood cells, can carry both oxygen and carbon dioxide, although in quite different ways. The decreased binding to carbon dioxide in the blood due to increased oxygen levels is known as the Haldane Effect, and is important in the transport of carbon dioxide from the tissues to the lungs. Conversely, a rise in the partial pressure of CO
2 or a lower pH will cause offloading of oxygen from hemoglobin. This is known as the Bohr Effect.
Carbon dioxide may be one of the mediators of local autoregulation of blood supply. If its levels are high, the capillaries expand to allow a greater blood flow to that tissue. [15]
Bicarbonate ions are crucial for regulating blood pH. A person's breathing rate influences the level of CO
2 in their blood. Breathing that is too slow or shallow can cause respiratory acidosis, while breathing that is too rapid may lead to hyperventilation, which may cause respiratory alkalosis.
Although the body requires oxygen for metabolism, low oxygen levels do not stimulate breathing. Rather, breathing is stimulated by higher carbon dioxide levels. As a result, breathing low-pressure air or a gas mixture with no oxygen at all (such as pure nitrogen) may lead to loss of consciousness. This is especially perilous for high-altitude fighter pilots. It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask to themselves first before helping others — otherwise one risks going unconscious without being aware of the imminent peril.
According to a study by the United States Department of Agriculture,[16] an average person's respiration generates approximately 450 liters (roughly 900 grams) of carbon dioxide per day.
See also
- Bosch reaction
- Carbon cycle
- Carbon dioxide (data page)
- Carbon dioxide sink
- CO
2 sensor - Carbon monoxide
- Emission standards
- Global warming
- Greenhouse gas
- Sabatier process
References
- ^ Staff (16 August 2006). "Carbon dioxide: IDLH Documentation". National Institute for Occupational Safety and Health. Retrieved 2007-07-05.
{{cite web}}: Check date values in:|date=(help) - ^ Santoro, M. (2006). "Amorphous silica-like carbon dioxide". Nature. 441 (7095): 857–860. doi:10.1038/nature04879. ISSN 0028-0836.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Priestley, Joseph (1772). "Observations on Different Kinds of Air". Philosophical Transactions. 62: 147–264. ISSN 0260-7085.
- ^ Davy, Humphry (1823). "On the Application of Liquids Formed by the Condensation of Gases as Mechanical Agents". Philosophical Transactions. 113: 199–205. ISSN 0261-0523.
- ^ Duane, H.D. Roller (1952). "Thilorier and the First Solidification of a "Permanent" Gas (1835)". Isis. 43 (2): 109–113. ISSN 0021-1753.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Pierantozzi, Ronald (2001). "Carbon Dioxide". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. doi:10.1002/0471238961.0301180216090518.a01.pub2.
- ^ Davidson, Sarah (Jan. 17, 2005). "Sweet and environmentally beneficial discovery: Plastics made from orange peel and a greenhouse gas". Cornell News. Retrieved 2007-09-09.
{{cite web}}: Check date values in:|date=(help); Cite has empty unknown parameter:|coauthors=(help) - ^ http://www.centrogalileo.it/nuovaPA/Articoli%20tecnici/INGLESE%20CONVEGNO/LA%20PORTE.doc
- ^ Coca-Cola beverage cooler
- ^ "Modine reinforces its CO2 research efforts". R744.com. 2007-06-28.
- ^ Sigurdsson, H. et al., (2000) Encyclopedia of Volcanoes, San Diego, Academic Press
- ^ "Volcanic Gases and Their Effects". Retrieved 2007-09-07.
- ^ Blom, T.J. (2002-12). "Carbon Dioxide In Greenhouses". Retrieved 2007-06-12.
{{cite web}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Falkowski, P. (2000). "The global carbon cycle: a test of our knowledge of earth as a system". Science. 290 (5490): 291–296. doi:10.1126/science.290.5490.291. ISSN 0036-8075.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hannan, Jerry. "Your Role in the "Greenhouse Effect"". Retrieved 2006-04-19.
External links
- Pressure-Temperature phase diagram for carbon dioxide
- Molview from bluerhinos.co.uk See Carbon dioxide in 3D
- Dry Ice information
- Trends in Atmospheric Carbon Dioxide (NOAA)
- Phase Diagram of Carbon Dioxide
- Experiment 071 -- Triple Point Phase Transition for Carbon Dioxide
- CO
2 as a natural refrigerant - FAQs

