Fossil fuel

Fossil fuels are fuels formed by natural resources such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years.[1] The fossil fuels include coal, petroleum, and natural gas which contain high percentages of carbon.
Fossil fuels range from volatile materials with low carbon:hydrogen ratios like methane, to liquid petroleum to nonvolatile materials composed of almost pure carbon, like anthracite coal. Methane can be found in hydrocarbon fields, alone, associated with oil, or in the form of methane clathrates. It is generally accepted that they formed from the fossilized remains of dead plants and animals[2] by exposure to heat and pressure in the Earth's crust over millions of years.[3] This biogenic theory was first introduced by Georg Agricola in 1556 and later by Mikhail Lomonosov in the 18th century.
It was estimated by the Energy Information Administration that in 2007 primary sources of energy consisted of petroleum 36.0%, coal 27.4%, natural gas 23.0%, amounting to an 86.4% share for fossil fuels in primary energy consumption in the world.[4]. Non-fossil sources in 2006 included hydroelectric 6.3%, nuclear 8.5%, and (geothermal, solar, tide, wind, wood, waste) amounting 0.9 percent.[5] World energy consumption was growing about 2.3% per year.
Fossil fuels are non-renewable resources because they take millions of years to form, and reserves are being depleted much faster than new ones are being made. The production and use of fossil fuels raise environmental concerns. A global movement toward the generation of renewable energy is therefore under way to help meet increased energy needs.
The burning of fossil fuels produces around 21.3 billion tonnes (21.3 gigatonnes) of carbon dioxide per year, but it is estimated that natural processes can only absorb about half of that amount, so there is a net increase of 10.65 billion tonnes of atmospheric carbon dioxide per year (one tonne of atmospheric carbon is equivalent to 44/12 or 3.7 tonnes of carbon dioxide).[6] Carbon dioxide is one of the greenhouse gases that enhances radiative forcing and contributes to global warming, causing the average surface temperature of the Earth to rise in response, which most climate scientists agree will cause major adverse effects.
Origin
Fossil fuels are formed by the anaerobic decomposition of remains of organisms including phytoplankton and zooplankton that settled to the sea (or lake) bottom in large quantities under anoxic conditions, millions of years ago. Over geological time, this organic matter, mixed with mud, got buried under heavy layers of sediment. The resulting high levels of heat and pressure caused the organic matter to chemically alter, first into a waxy material known as kerogen which is found in oil shales, and then with more heat into liquid and gaseous hydrocarbons in a process known as catagenesis.
There is a wide range of organic, or hydrocarbon, compounds in any given fuel mixture. The specific mixture of hydrocarbons gives a fuel its characteristic properties, such as boiling point, melting point, density, viscosity, etc. Some fuels like natural gas, for instance, contain only very low boiling, gaseous components. Others such as gasoline or diesel contain much higher boiling components.
Terrestrial plants, on the other hand, tend to form coal and methane. Many of the coal fields date to the Carboniferous period of Earth's history. Terrestrial plants also form type III kerogen, a source of natural gas.
Importance


Fossil fuels are of great importance because they can be burned (oxidized to carbon dioxide and water), producing significant amounts of energy. The use of coal as a fuel predates recorded history. Coal was used to run furnaces for the melting of metal ore. Semi-solid hydrocarbons from seeps were also burned in ancient times,[7] but these materials were mostly used for waterproofing and embalming.[8]
Commercial exploitation of petroleum, largely as a replacement for oils from animal sources (notably whale oil) for use in oil lamps began in the nineteenth century.[9]
Natural gas, once flared-off as an un-needed byproduct of petroleum production, is now considered a very valuable resource.[10]
Heavy crude oil, which is much more viscous than conventional crude oil, and tar sands, where bitumen is found mixed with sand and clay, are becoming more important as sources of fossil fuel.[11] Oil shale and similar materials are sedimentary rocks containing kerogen, a complex mixture of high-molecular weight organic compounds, which yield synthetic crude oil when heated (pyrolyzed). These materials have yet to be exploited commercially.[12] These fuels are employed in internal combustion engines, fossil fuel power stations and other uses.
Prior to the latter half of the eighteenth century, windmills or watermills provided the energy needed for industry such as milling flour, sawing wood or pumping water, and burning wood or peat provided domestic heat. The wide-scale use of fossil fuels, coal at first and petroleum later, to fire steam engines, enabled the Industrial Revolution. At the same time, gas lights using natural gas or coal gas were coming into wide use. The invention of the internal combustion engine and its use in automobiles and trucks greatly increased the demand for gasoline and diesel oil, both made from fossil fuels. Other forms of transportation, railways and aircraft also required fossil fuels. The other major use for fossil fuels is in generating electricity and the petrochemical industry. Tar, a leftover of petroleum extraction, is used in construction of roads.
Levels and flows
Levels of primary energy sources are the reserves in the ground. Flows are production. The most important part of primary energy sources are the carbon based fossil energy sources. Coal, oil, and natural gas provided 79.6% of primary energy production during 2002 (in million tonnes of oil equivalent (mtoe)) (34.9+23.5+21.2).
Levels (proved reserves) during 2005-2007
- Coal: 997,748 million short tonnes (905 billion metric tonnes),[13] 4,416 billion barrels of oil equivalent
- Oil: 1,119-1,317 billion barrels (178-209 billion kilolitres)[14]
- Natural gas: 6,183-6,381 trillion cubic feet (175-181 trillion cubic metres),[14] 1,161 billion barrels of oil equivalent
Flows (daily production) during 2006
- Coal: 18,476,127 short tonnes (16,761,260 metric tonnes),[15] 52 million barrels of oil equivalent per day
- Oil: 84 million barrels per day (13 million kilolitres)[16]
- Natural gas: 104,435 billion cubic feet (2,960 billion cubic metres),[17] 19 million barrels of oil equivalent per day
Years of production left in the ground with the current proved reserves and flows above
- Coal: 148 years
- Oil: 43 years
- Natural gas: 61 years
Years of production left in the ground with the most optimistic proved reserve estimates (Oil & Gas Journal, World Oil)[citation needed]
- Coal: 417 years
- Oil: 43 years
- Natural gas: 167 years
The calculation above assumes that the product could be produced at a constant level for that number of years and that all of the proved reserves could be recovered. In reality, consumption of all three resources has been increasing. While this suggests that the resource will be used up more quickly, in reality, the production curve is much more akin to a bell curve. At some point in time, the production of each resource within an area, country, or globally will reach a maximum value, after which, the production will decline until it reaches a point where is no longer economically feasible or physically possible to produce. See Hubbert peak theory for detail on this decline curve with regard to petroleum. Note also that proved reserve estimates do not include strategic reserves, which (globally) amount to 4.1 billion more barrels.
The above discussion emphasizes worldwide energy balance. It is also valuable to understand the ratio of reserves to annual consumption (R/C) by region or country. For example, energy policy of the United Kingdom recognizes that Europe's R/C value is 3.0, very low by world standards, and exposes that region to energy vulnerability. Alternatives to fossil fuels are a subject of intense debate worldwide.
Limits and alternatives
The principle of supply and demand suggests that as hydrocarbon supplies diminish, prices will rise. Therefore higher prices will lead to increased alternative, renewable energy supplies as previously uneconomic sources become sufficiently economical to exploit. Artificial gasolines and other renewable energy sources currently require more expensive production and processing technologies than conventional petroleum reserves, but may become economically viable in the near future. See Energy development. Different alternative sources of energy include nuclear, hydroelectric, solar, wind, and geothermal.
Environmental effects
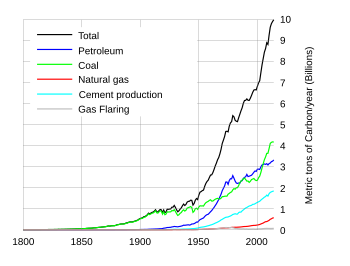
In the United States, more than 90% of greenhouse gas emissions come from the combustion of fossil fuels.[18] Combustion of fossil fuels also produces other air pollutants, such as nitrogen oxides, sulphur dioxide, volatile organic compounds and heavy metals.
According to Environment Canada:
"The electricity sector is unique among industrial sectors in its very large contribution to emissions associated with nearly all air issues. Electricity generation produces a large share of Canadian nitrogen oxides and sulphur dioxide emissions, which contribute to smog and acid rain and the formation of fine particulate matter. It is the largest uncontrolled industrial source of mercury emissions in Canada. Fossil fuel-fired electric power plants also emit carbon dioxide, which may contribute to climate change. In addition, the sector has significant impacts on water and habitat and species. In particular, hydro dams and transmission lines have significant effects on water and biodiversity."[19]

According to U.S. Scientist Jerry Mahlman and USA Today: Mahlman, who crafted the IPCC language used to define levels of scientific certainty, says the new report will lay the blame at the feet of fossil fuels with "virtual certainty," meaning 99% sure. That's a significant jump from "likely," or 66% sure, in the group's last report in 2001, Mahlman says. His role in this year's effort involved spending two months reviewing the more than 1,600 pages of research that went into the new assessment.[20]
Combustion of fossil fuels generates sulfuric, carbonic, and nitric acids, which fall to Earth as acid rain, impacting both natural areas and the built environment. Monuments and sculptures made from marble and limestone are particularly vulnerable, as the acids dissolve calcium carbonate.
Fossil fuels also contain radioactive materials, mainly uranium and thorium, which are released into the atmosphere. In 2000, about 12,000 tonnes of thorium and 5,000 tonnes of uranium were released worldwide from burning coal.[21] It is estimated that during 1982, US coal burning released 155 times as much radioactivity into the atmosphere as the Three Mile Island incident.[22] However, this radioactivity from coal burning is minuscule at each source and has not shown to have any adverse effect on human physiology.[citation needed]
Burning coal also generates large amounts of bottom ash and fly ash. These materials are used in a wide variety of applications, utilizing, for example, about 40% of the US production.[23]
Harvesting, processing, and distributing fossil fuels can also create environmental concerns. Coal mining methods, particularly mountaintop removal and strip mining, have negative environmental impacts, and offshore oil drilling poses a hazard to aquatic organisms. Oil refineries also have negative environmental impacts, including air and water pollution. Transportation of coal requires the use of diesel-powered locomotives, while crude oil is typically transported by tanker ships, each of which requires the combustion of additional fossil fuels.
Environmental regulation uses a variety of approaches to limit these emissions, such as command-and-control (which mandates the amount of pollution or the technology used), economic incentives, or voluntary programs.
An example of such regulation in the USA is the "EPA is implementing policies to reduce airborne mercury emissions. Under regulations issued in 2005, coal-fired power plants will need to reduce their emissions by 70 percent by 2018."[24].
In economic terms, pollution from fossil fuels is regarded as a negative externality. Taxation is considered one way to make societal costs explicit, in order to 'internalize' the cost of pollution. This aims to make fossil fuels more expensive, thereby reducing their use and the amount of pollution associated with them, along with raising the funds necessary to counteract these factors.
Former CIA Director James Woolsey recently outlined the national security arguments in favor of moving away from fossil fuels.[25]
See also
- Abiogenic petroleum origin proposes that petroleum is not a fossil fuel
- C. Arden Pope
- Fossil Fools Day
- Fossil fuel drilling
- Fossil fuel exporters
- Carbon based fuel
- Clinker (waste)
- Oil reserves
- Petroleum
- Radiative forcing
- Upstream and downstream
References
- ^ Paul Mann, Lisa Gahagan, and Mark B. Gordon, "Tectonic setting of the world's giant oil and gas fields," in Michel T. Halbouty (ed.) Giant Oil and Gas Fields of the Decade, 1990-1999, Tulsa, Okla.: American Association of Petroleum Geologists, p.50, accessed 22 June 2009.
- ^ Dr. Irene Novaczek. "Canada's Fossil Fuel Dependency". Elements. Retrieved 2007-01-18.
- ^ "Fossil fuel". EPA. Retrieved 2007-01-18.
- ^ "U.S. EIA International Energy Statistics". Retrieved 2010-01-12.
- ^ "International Energy Annual 2006". Retrieved 2009-02-08.
- ^ "US Department of Energy on greenhouse gases". Retrieved 2007-09-09.
- ^ "Encyclopedia Britannica, use of oil seeps in accient times". Retrieved 2007-09-09.
- ^ Bilkadi, Zayn (1994). "BULLS FROM THE SEA : Ancient Oil Industries" ([dead link]). Aramco World. Retrieved 2007-09-09.
- ^ Ball, Max W. (1965). This Fascinating Oil Business. Indianapolis: Bobbs-Merrill. ISBN 0-672-50829-X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kaldany,, Rashad, Director Oil, Gas, Mining and Chemicals Dept, World Bank (December 13, 2006). Global Gas Flaring Reduction: A Time for Action! (PDF). Global Forum on Flaring & Gas Utilization. Paris. Retrieved 2007-09-09.
{{cite conference}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ "Oil Sands Global Market Potential 2007". Retrieved 2007-09-09.
- ^ "US Department of Energy plans for oil shale development". Retrieved 2007-09-09.
- ^ World Estimated Recoverable Coal
- ^ a b World Proved Reserves of Oil and Natural Gas, Most Recent Estimates
- ^ http://www.eia.doe.gov/pub/international/iealf/table14.xls
- ^ http://www.eia.doe.gov/emeu/international/RecentPetroleumConsumptionBarrelsperDay.xls
- ^ http://www.eia.doe.gov/pub/international/iealf/table13.xls
- ^ US EPA.2000. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-1998, Rep. EPA 236-R-00-01. US EPA, Washington, DC, http://www.epa.gov/globalwarming
- ^ "Electricity Generation". Retrieved 2007-03-23.
- ^ O'Driscoll, Patrick; Vergano, Dan (2007-03-01). "Fossil fuels are to blame, world scientists conclude". USA Today. Retrieved 2010-05-02.
- ^ Coal Combustion: Nuclear Resource or Danger - Alex Gabbard
- ^ Nuclear proliferation through coal burning - Gordon J. Aubrecht, II, Ohio State University
- ^ American Coal Ash Association. ""CCP Production and Use Survey"" (PDF).
- ^ "Frequently Asked Questions, Information on Proper Disposal of Compact Fluorescent Light Bulbs (CFLs)" (PDF). Retrieved 2007-03-19.
- ^ Video of Woolsey speech
External links
- "The Coming Energy Crisis?" - essay by James L. Williams of WTRG Economics and A. F. Alhajji of Ohio Northern University
- "Powering the Future" - Michael Parfit (National Geographic)
- "Federal Fossil Fuel Subsidies and Greenhouse Gas Emissions"
- Fossil Fuel Subsidies in Europe
- Oil companies hit by 'state' cyber attacks
Debate
- The Origin of Methane (and Oil) in the Crust of the Earth-Thomas Gold (Internet Archives)
