Malaria
| Malaria | |
|---|---|
| Specialty | Infectious diseases, tropical medicine, parasitology |
Malaria is a mosquito-borne infectious disease of humans caused by eukaryotic protists of the genus Plasmodium. It is widespread in tropical and subtropical regions, including much of Sub-Saharan Africa, Asia and the Americas. The disease results from the multiplication of malaria parasites within red blood cells, causing symptoms that typically include fever and headache, in severe cases progressing to coma, and death.
Four species of Plasmodium can infect and be transmitted by humans. Severe disease is largely caused by Plasmodium falciparum. Malaria caused by Plasmodium vivax, Plasmodium ovale and Plasmodium malariae is generally a milder disease that is rarely fatal. A fifth species, Plasmodium knowlesi, is a zoonosis that causes malaria in macaques but can also infect humans.[1][2]
Malaria transmission can be reduced by preventing mosquito bites by distribution of inexpensive mosquito nets and insect repellents, or by mosquito-control measures such as spraying insecticides inside houses and draining standing water where mosquitoes lay their eggs. Although many are under development, the challenge of producing a widely available vaccine that provides a high level of protection for a sustained period is still to be met.[3] Two drugs are also available to prevent malaria in travellers to malaria-endemic countries (prophylaxis).
A variety of antimalarial medications are available. In the last 5 years, treatment of P. falciparum infections in endemic countries has been transformed by the use of combinations of drugs containing an artemisinin derivative. Severe malaria is treated with intravenous or intramuscular quinine or, increasingly, the artemisinin derivative artesunate [4] which is superior to quinine in both children and adults.[5] Resistance has developed to several antimalarial drugs, most notably chloroquine.[6]
Each year, there are more than 225 million cases of malaria,[7] killing around 781,000 people each year according to the latest WHO Report, 2.23% of deaths worldwide. The majority of deaths are of young children in sub-Saharan Africa.[8] Ninety percent of malaria-related deaths occur in sub-Saharan Africa. Malaria is commonly associated with poverty, and can indeed be a cause of poverty[9] and a major hindrance to economic development.
It has been estimated that malaria has caused half of all human deaths throughout human history. [10]
Signs and symptoms
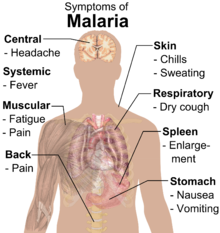

Symptoms of malaria include fever, shivering, arthralgia (joint pain), vomiting, anemia (caused by hemolysis), hemoglobinuria, retinal damage,[12] and convulsions. The classic symptom of malaria is cyclical occurrence of sudden coldness followed by rigor and then fever and sweating lasting four to six hours, occurring every two days in P. vivax and P. ovale infections, while every three days for P. malariae.[13] P. falciparum can have recurrent fever every 36–48 hours or a less pronounced and almost continuous fever. For reasons that are poorly understood, but that may be related to high intracranial pressure, children with malaria frequently exhibit abnormal posturing, a sign indicating severe brain damage.[14] Malaria has been found to cause cognitive impairments, especially in children. It causes widespread anemia during a period of rapid brain development and also direct brain damage. This neurologic damage results from cerebral malaria to which children are more vulnerable.[15][16] Cerebral malaria is associated with retinal whitening,[17] which may be a useful clinical sign in distinguishing malaria from other causes of fever.[18]
Severe malaria is almost exclusively caused by P. falciparum infection, and usually arises 6–14 days after infection.[19] Consequences of severe malaria include coma and death if untreated—young children and pregnant women are especially vulnerable. Splenomegaly (enlarged spleen), severe headache, cerebral ischemia, hepatomegaly (enlarged liver), hypoglycemia, and hemoglobinuria with renal failure may occur. Renal failure is a feature of blackwater fever, where hemoglobin from lysed red blood cells leaks into the urine. Severe malaria can progress extremely rapidly and cause death within hours or days.[19] In the most severe cases of the disease, fatality rates can exceed 20%, even with intensive care and treatment.[20] In endemic areas, treatment is often less satisfactory and the overall fatality rate for all cases of malaria can be as high as one in ten.[21] Over the longer term, developmental impairments have been documented in children who have suffered episodes of severe malaria.[22]
Cause

Malaria parasites are members of the genus Plasmodium (phylum Apicomplexa). In humans malaria is caused by P. falciparum, P. malariae, P. ovale, P. vivax and P. knowlesi.[23][24] P. falciparum is the most common cause of infection and is responsible for about 80% of all malaria cases, and is also responsible for about 90% of the deaths from malaria.[25] Parasitic Plasmodium species also infect birds, reptiles, monkeys, chimpanzees and rodents.[26] There have been documented human infections with several simian species of malaria, namely P. knowlesi, P. inui, P. cynomolgi,[27] P. simiovale, P. brazilianum, P. schwetzi and P. simium; however, with the exception of P. knowlesi, these are mostly of limited public health importance.[28]
Malaria parasites contain apicoplasts, an organelle usually found in plants, complete with their own functioning genomes. These apicoplast are thought to have originated through the endosymbiosis of algae[29] and play a crucial role in various aspects of parasite metabolism e.g. fatty acid bio-synthesis.[30] To date, 466 proteins have been found to be produced by apicoplasts[31] and these are now being looked at as possible targets for novel anti-malarial drugs.
Life cycle
The parasite's secondary (intermediate) hosts are humans and other vertebrates. Female mosquitoes of the Anopheles genus are primary hosts and transmission vectors. Young mosquitoes first ingest the malaria parasite by feeding on an infected human carrier and the infected Anopheles mosquitoes carry Plasmodium sporozoites in their salivary glands. A mosquito becomes infected when it takes a blood meal from an infected human. Once ingested, the parasite gametocytes taken up in the blood will further differentiate into male or female gametes and then fuse in the mosquito's gut. This produces an ookinete that penetrates the gut lining and produces an oocyst in the gut wall. When the oocyst ruptures, it releases sporozoites that migrate through the mosquito's body to the salivary glands, where they are then ready to infect a new human host. This type of transmission is occasionally referred to as anterior station transfer.[32] The sporozoites are injected into the skin, alongside saliva, when the mosquito takes a subsequent blood meal.
Only female mosquitoes feed on blood while male mosquitoes feed on plant nectar,[33] thus males do not transmit the disease. The females of the Anopheles genus of mosquito prefer to feed at night. They usually start searching for a meal at dusk, and will continue throughout the night until taking a meal. Malaria parasites can also be transmitted by blood transfusions, although this is rare.[34]
Recurrent malaria
Malaria recurs after treatment for three reasons. Recrudescence occurs when parasites are not cleared by treatment, whereas reinfection indicates complete clearance with new infection established from a separate infective mosquito bite; both can occur with any malaria parasite species. Relapse is specific to P. vivax and P. ovale and involves re-emergence of blood-stage parasites from latent parasites (hypnozoites) in the liver. Describing a case of malaria as cured by observing the disappearance of parasites from the bloodstream can, therefore, be deceptive. The longest incubation period reported for a P. vivax infection is 30 years.[19] Approximately one in five of P. vivax malaria cases in temperate areas involve overwintering by hypnozoites (i.e., relapses begin the year after the mosquito bite).[35]
Pathogenesis

Malaria develops via two phases: an exoerythrocytic and an erythrocytic phase. The exoerythrocytic phase involves infection of the hepatic system, or liver, whereas the erythrocytic phase involves infection of the erythrocytes, or red blood cells. When an infected mosquito pierces a person's skin to take a blood meal, sporozoites in the mosquito's saliva enter the bloodstream and migrate to the liver. Within minutes of being introduced into the human host, the sporozoites infect hepatocytes, multiplying asexually and asymptomatically for a period of 8–30 days.[36] Once in the liver, these organisms differentiate to yield thousands of merozoites, which, following rupture of their host cells, escape into the blood and infect red blood cells, thus beginning the erythrocytic stage of the life cycle.[36] The parasite escapes from the liver undetected by wrapping itself in the cell membrane of the infected host liver cell.[37]
Within the red blood cells, the parasites multiply further, again asexually, periodically breaking out of their hosts to invade fresh red blood cells. Several such amplification cycles occur. Thus, classical descriptions of waves of fever arise from simultaneous waves of merozoites escaping and infecting red blood cells.
Some P. vivax and P. ovale sporozoites do not immediately develop into exoerythrocytic-phase merozoites, but instead produce hypnozoites that remain dormant for periods ranging from several months (6–12 months is typical) to as long as three years. After a period of dormancy, they reactivate and produce merozoites. Hypnozoites are responsible for long incubation and late relapses in these two species of malaria.[38]
The parasite is relatively protected from attack by the body's immune system because for most of its human life cycle it resides within the liver and blood cells and is relatively invisible to immune surveillance. However, circulating infected blood cells are destroyed in the spleen. To avoid this fate, the P. falciparum parasite displays adhesive proteins on the surface of the infected blood cells, causing the blood cells to stick to the walls of small blood vessels, thereby sequestering the parasite from passage through the general circulation and the spleen.[39] This "stickiness" is the main factor giving rise to hemorrhagic complications of malaria. High endothelial venules (the smallest branches of the circulatory system) can be blocked by the attachment of masses of these infected red blood cells. The blockage of these vessels causes symptoms such as in placental and cerebral malaria. In cerebral malaria the sequestrated red blood cells can breach the blood brain barrier possibly leading to coma.[40]
Although the white blood cell surface adhesive proteins (called PfEMP1, for Plasmodium falciparum erythrocyte membrane protein 1) are exposed to the immune system, they do not serve as good immune targets, because of their extreme diversity; there are at least 60 variations of the protein within a single parasite and effectively limitless versions within parasite populations.[39] The parasite switches between a broad repertoire of PfEMP17 surface proteins, thus staying one step ahead of the pursuing immune system.
Some merozoites turn into male and female gametocytes. Since the gametocytes are formed in the blood of the vertebrate host, the vertebrate host is the definitive host of the disease. If a mosquito pierces the skin of an infected person, it potentially picks up gametocytes within the blood. Fertilization and sexual recombination of the parasite occurs in the mosquito's gut. New sporozoites develop and travel to the mosquito's salivary gland, completing the cycle. Pregnant women are especially attractive to the mosquitoes,[41] and malaria in pregnant women is an important cause of stillbirths, infant mortality and low birth weight,[42] particularly in P. falciparum infection, but also in other species infection, such as P. vivax.[43]
Genetic resistance
Malaria is thought to have been the greatest selective pressure on the human genome in recent history.[44] This is due to the high levels of mortality and morbidity caused by malaria, especially the P. falciparum species. A number of diseases may provide some resistance to it including sickle cell disease, thalassaemias, glucose-6-phosphate dehydrogenase, Duffy antigens, and possibly others.
Diagnosis
The mainstay of malaria diagnosis has been the microscopic examination of blood.[45] Although blood is the sample most frequently used to make a diagnosis, both saliva and urine have been investigated as alternative, less invasive specimens.[46]
Areas that cannot afford laboratory diagnostic tests often use only a history of subjective fever as the indication to treat for malaria. Using Giemsa-stained blood smears from children in Malawi, one study showed that when clinical predictors (rectal temperature, nailbed pallor, and splenomegaly) were used as treatment indications, rather than using only a history of subjective fevers, a correct diagnosis increased from 2% to 41% of cases, and unnecessary treatment for malaria was significantly decreased.[47]
Blood films
| Species | Appearance | Periodicity | Liver persistent |
|---|---|---|---|
| Plasmodium vivax |  |
tertian | yes |
| Plasmodium ovale |  |
tertian | yes |
| Plasmodium falciparum |  |
tertian | no |
| Plasmodium malariae | 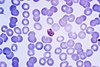 |
quartan | no |
The most economic, preferred, and reliable diagnosis of malaria is microscopic examination of blood films because each of the four major parasite species has distinguishing characteristics. Two sorts of blood film are traditionally used. Thin films are similar to usual blood films and allow species identification because the parasite's appearance is best preserved in this preparation. Thick films allow the microscopist to screen a larger volume of blood and are about eleven times more sensitive than the thin film, so picking up low levels of infection is easier on the thick film, but the appearance of the parasite is much more distorted and therefore distinguishing between the different species can be much more difficult. With the pros and cons of both thick and thin smears taken into consideration, it is imperative to utilize both smears while attempting to make a definitive diagnosis.[48]
From the thick film, an experienced microscopist can detect parasite levels (or parasitemia) down to as low as 0.0000001% of red blood cells. Diagnosis of species can be difficult because the early trophozoites ("ring form") of all four species look identical and it is never possible to diagnose species on the basis of a single ring form; species identification is always based on several trophozoites.
One important thing to note is that P. malariae and P. knowlesi (which is the most common cause of malaria in South-east Asia) look very similar under the microscope. However, P. knowlesi parasitemia increases very fast and causes more severe disease than P. malariae, so it is important to identify and treat infections quickly. Therefore modern methods such as PCR (see "Molecular methods" below) or monoclonal antibody panels that can distinguish between the two should be used in this part of the world.[49]
Antigen tests
For areas where microscopy is not available, or where laboratory staff are not experienced at malaria diagnosis, there are commercial antigen detection tests that require only a drop of blood.[50] Immunochromatographic tests (also called: Malaria Rapid Diagnostic Tests, Antigen-Capture Assay or "Dipsticks") have been developed, distributed and fieldtested. These tests use finger-stick or venous blood, the completed test takes a total of 15–20 minutes, and the results are read visually as the presence or absence of colored stripes on the dipstick, so they are suitable for use in the field. The threshold of detection by these rapid diagnostic tests is in the range of 100 parasites/µl of blood (commercial kits can range from about 0.002% to 0.1% parasitemia) compared to 5 by thick film microscopy. One disadvantage is that dipstick tests are qualitative but not quantitative – they can determine if parasites are present in the blood, but not how many.
The first rapid diagnostic tests were using P. falciparum glutamate dehydrogenase as antigen.[51] PGluDH was soon replaced by P.falciparum lactate dehydrogenase, a 33 kDa oxidoreductase [EC 1.1.1.27]. It is the last enzyme of the glycolytic pathway, essential for ATP generation and one of the most abundant enzymes expressed by P.falciparum. PLDH does not persist in the blood but clears about the same time as the parasites following successful treatment. The lack of antigen persistence after treatment makes the pLDH test useful in predicting treatment failure. In this respect, pLDH is similar to pGluDH. Depending on which monoclonal antibodies are used, this type of assay can distinguish between all five different species of human malaria parasites, because of antigenic differences between their pLDH isoenzymes.
Molecular methods
Molecular methods are available in some clinical laboratories and rapid real-time assays (for example, QT-NASBA based on the polymerase chain reaction)[52] are being developed with the hope of being able to deploy them in endemic areas.
PCR (and other molecular methods) is more accurate than microscopy. However, it is expensive, and requires a specialized laboratory. Moreover, levels of parasitemia are not necessarily correlative with the progression of disease, particularly when the parasite is able to adhere to blood vessel walls. Therefore more sensitive, low-tech diagnosis tools need to be developed in order to detect low levels of parasitemia in the field.[53]
Differential
Fever and septic shock are commonly misdiagnosed as severe malaria in Africa, leading to a failure to treat other life-threatening illnesses. In malaria-endemic areas, parasitemia does not ensure a diagnosis of severe malaria, because parasitemia can be incidental to other concurrent disease. Recent investigations suggest that malarial retinopathy is better (collective sensitivity of 95% and specificity of 90%) than any other clinical or laboratory feature in distinguishing malarial from non-malarial coma.[54]
Prevention

Methods used in order to prevent the spread of disease, or to protect individuals in areas where malaria is endemic, include prophylactic drugs, mosquito eradication and the prevention of mosquito bites.
The continued existence of malaria in an area requires a combination of high human population density, high mosquito population density and high rates of transmission from humans to mosquitoes and from mosquitoes to humans. If any of these is lowered sufficiently, the parasite will sooner or later disappear from that area, as happened in North America, Europe and much of Middle East. However, unless the parasite is eliminated from the whole world, it could become re-established if conditions revert to a combination that favours the parasite's reproduction.[citation needed] Many countries are seeing an increasing number of imported malaria cases owing to extensive travel and migration.
Many researchers argue that prevention of malaria may be more cost-effective than treatment of the disease in the long run, but the capital costs required are out of reach of many of the world's poorest people. Economic adviser Jeffrey Sachs estimates that malaria can be controlled for US$3 billion in aid per year.[55]
A 2008 study that examined international financing of malaria control found large regional variations in the levels of average annual per capita funding ranging from US$0.01 in Myanmar to US$147 in Suriname. The study found 34 countries where the funding was less than US$1 per capita, including 16 countries where annual malaria support was less than US$0.5. The 16 countries included 710 million people or 50% of the global population exposed to the risks of malaria transmission, including seven of the poorest countries in Africa (Côte d'Ivoire, Republic of the Congo, Chad, Mali, Democratic Republic of the Congo, Somalia, and Guinea) and two of the most densely populated stable endemic countries in the world (Indonesia and India).[56]
Brazil, Eritrea, India, and Vietnam, unlike many other developing nations, have successfully reduced the malaria burden. Common success factors have included conducive country conditions, a targeted technical approach using a package of effective tools, data-driven decision-making, active leadership at all levels of government, involvement of communities, decentralized implementation and control of finances, skilled technical and managerial capacity at national and sub-national levels, hands-on technical and programmatic support from partner agencies, and sufficient and flexible financing.[57]
Medications
Several drugs, most of which are also used for treatment of malaria, can be taken preventively. Modern drugs used include mefloquine (Lariam), doxycycline (available generically), and the combination of atovaquone and proguanil hydrochloride (Malarone). Doxycycline and the atovaquone and proguanil combination are the best tolerated with mefloquine associated with higher rates of neurological and psychiatric symptoms.[58] The choice of which drug to use depends on which drugs the parasites in the area are resistant to, as well as side-effects and other considerations. The prophylactic effect does not begin immediately upon starting taking the drugs, so people temporarily visiting malaria-endemic areas usually begin taking the drugs one to two weeks before arriving and must continue taking them for 4 weeks after leaving (with the exception of atovaquone proguanil that only needs be started 2 days prior and continued for 7 days afterwards). Generally, these drugs are taken daily or weekly, at a lower dose than would be used for treatment of a person who had actually contracted the disease. Use of prophylactic drugs is seldom practical for full-time residents of malaria-endemic areas, and their use is usually restricted to short-term visitors and travelers to malarial regions. This is due to the cost of purchasing the drugs, negative side effects from long-term use, and because some effective anti-malarial drugs are difficult to obtain outside of wealthy nations.
Quinine was used historically, however the development of more effective alternatives such as quinacrine, chloroquine, and primaquine in the 20th century reduced its use. Today, quinine is not generally used for prophylaxis. The use of prophylactic drugs where malaria-bearing mosquitoes are present may encourage the development of partial immunity.[59]
Vector control
Efforts to eradicate malaria by eliminating mosquitoes have been successful in some areas. Malaria was once common in the United States and southern Europe, but vector control programs, in conjunction with the monitoring and treatment of infected humans, eliminated it from those regions. In some areas, the draining of wetland breeding grounds and better sanitation were adequate. Malaria was eliminated from most parts of the USA in the early 20th century by such methods, and the use of the pesticide DDT and other means eliminated it from the remaining pockets in the South by 1951[60] (see National Malaria Eradication Program). In 2002, there were 1,059 cases of malaria reported in the US, including eight deaths, but in only five of those cases was the disease contracted in the United States.
Before DDT, malaria was successfully eradicated or controlled also in several tropical areas by removing or poisoning the breeding grounds of the mosquitoes or the aquatic habitats of the larva stages, for example by filling or applying oil to places with standing water. These methods have seen little application in Africa for more than half a century.[61]
Sterile insect technique is emerging as a potential mosquito control method. Progress towards transgenic, or genetically modified, insects suggest that wild mosquito populations could be made malaria-resistant. Researchers at Imperial College London created the world's first transgenic malaria mosquito,[62] with the first plasmodium-resistant species announced by a team at Case Western Reserve University in Ohio in 2002.[63] Successful replacement of current populations with a new genetically modified population, relies upon a drive mechanism, such as transposable elements to allow for non-Mendelian inheritance of the gene of interest. However, this approach contains many difficulties and success is a distant prospect.[64] An even more futuristic method of vector control is the idea that lasers could be used to kill flying mosquitoes.[65]
Indoor residual spraying
Indoor residual spraying (IRS) is the practice of spraying insecticides on the interior walls of homes in malaria affected areas. After feeding, many mosquito species rest on a nearby surface while digesting the bloodmeal, so if the walls of dwellings have been coated with insecticides, the resting mosquitos will be killed before they can bite another victim, transferring the malaria parasite.
The first pesticide used for IRS was DDT.[60] Although it was initially used exclusively to combat malaria, its use quickly spread to agriculture. In time, pest-control, rather than disease-control, came to dominate DDT use, and this large-scale agricultural use led to the evolution of resistant mosquitoes in many regions. The DDT resistance shown by Anopheles mosquitoes can be compared to antibiotic resistance shown by bacteria. The overuse of anti-bacterial soaps and antibiotics led to antibiotic resistance in bacteria, similar to how overspraying of DDT on crops led to DDT resistance in Anopheles mosquitoes. During the 1960s, awareness of the negative consequences of its indiscriminate use increased, ultimately leading to bans on agricultural applications of DDT in many countries in the 1970s. Since the use of DDT has been limited or banned for agricultural use for some time, DDT may now be more effective as a method of disease-control.
Although DDT has never been banned for use in malaria control and there are several other insecticides suitable for IRS, some advocates have claimed that bans are responsible for tens of millions of deaths in tropical countries where DDT had once been effective in controlling malaria. Furthermore, most of the problems associated with DDT use stem specifically from its industrial-scale application in agriculture, rather than its use in public health.[66]
The World Health Organization (WHO) currently advises the use of 12 different insecticides in IRS operations, including DDT as well as alternative insecticides (such as the pyrethroids permethrin and deltamethrin).[67] This public health use of small amounts of DDT is permitted under the Stockholm Convention on Persistent Organic Pollutants (POPs), which prohibits the agricultural use of DDT.[68] However, because of its legacy, many developed countries previously discouraged DDT use even in small quantities.[69]
One problem with all forms of Indoor Residual Spraying is insecticide resistance via evolution of mosquitos. According to a study published on Mosquito Behavior and Vector Control, mosquito species that are affected by IRS are endophilic species (species that tend to rest and live indoors), and due to the irritation caused by spraying, their evolutionary descendants are trending towards becoming exophilic (species that tend to rest and live out of doors), meaning that they are not as affected—if affected at all—by the IRS, rendering it somewhat useless as a defense mechanism.[70]
Mosquito nets and bedclothes
Mosquito nets help keep mosquitoes away from people and greatly reduce the infection and transmission of malaria. The nets are not a perfect barrier and they are often treated with an insecticide designed to kill the mosquito before it has time to search for a way past the net. Insecticide-treated nets (ITNs) are estimated to be twice as effective as untreated nets and offer greater than 70% protection compared with no net.[71] Although ITNs are proven to be very effective against malaria, less than 2% of children in urban areas in Sub-Saharan Africa are protected by ITNs. Since the Anopheles mosquitoes feed at night, the preferred method is to hang a large "bed net" above the center of a bed such that it drapes down and covers the bed completely.
Vaccination
Immunity (or, more accurately, tolerance) does occur naturally, but only in response to repeated infection with multiple strains of malaria.[72] Vaccines for malaria are under development, with no completely effective vaccine yet available. The first promising studies demonstrating the potential for a malaria vaccine were performed in 1967 by immunizing mice with live, radiation-attenuated sporozoites, providing protection to about 60% of the mice upon subsequent injection with normal, viable sporozoites.[73] Since the 1970s, there has been a considerable effort to develop similar vaccination strategies within humans. It was determined that an individual can be protected from a P. falciparum infection if they receive over 1,000 bites from infected yet irradiated mosquitoes.[74]
Other methods
Education in recognizing the symptoms of malaria has reduced the number of cases in some areas of the developing world by as much as 20%. Recognizing the disease in the early stages can also stop the disease from becoming a killer. Education can also inform people to cover over areas of stagnant, still water e.g. Water Tanks which are ideal breeding grounds for the parasite and mosquito, thus cutting down the risk of the transmission between people. This is most put in practice in urban areas where there are large centers of population in a confined space and transmission would be most likely in these areas.
The Malaria Control Project is currently using downtime computing power donated by individual volunteers around the world (see Volunteer computing and BOINC) to simulate models of the health effects and transmission dynamics in order to find the best method or combination of methods for malaria control. This modeling is extremely computer intensive due to the simulations of large human populations with a vast range of parameters related to biological and social factors that influence the spread of the disease. It is expected to take a few months using volunteered computing power compared to the 40 years it would have taken with the current resources available to the scientists who developed the program.[75]
An example of the importance of computer modeling in planning malaria eradication programs is shown in the paper by Águas and others. They showed that eradication of malaria is crucially dependent on finding and treating the large number of people in endemic areas with asymptomatic malaria, who act as a reservoir for infection.[76] The malaria parasites do not affect animal species and therefore eradication of the disease from the human population would be expected to be effective.
Other interventions for the control of malaria include mass drug administrations and intermittent preventive therapy.
Furthering attempts to reduce transmission rates, a proposed alternative to mosquito nets is the mosquito laser, or photonic fence, which identifies female mosquitoes and shoots them using a medium-powered laser.[77] The device is currently undergoing commercial development, although instructions for a DIY version of the photonic fence have also been published.[78]
Another way of reducing the malaria transmitted to humans from mosquitoes has been developed by the University of Arizona. They have engineered a mosquito to become resistant to malaria. This was reported on the 16 July 2010 in the journal PLoS Pathogens.[79] With the ultimate end being that the release of this GM mosquito into the environment, Gareth Lycett, a malaria researcher from Liverpool School of Tropical Medicine told the BBC that "It is another step on the journey towards potentially assisting malaria control through GM mosquito release."[80]
Treatment
When properly treated, a patient with malaria can expect a complete recovery.[81] The treatment of malaria depends on the severity of the disease; whether patients who can take oral drugs have to be admitted depends on the assessment and the experience of the clinician. Uncomplicated malaria is treated with oral drugs. The most effective strategy for P. falciparum infection recommended by WHO is the use of artemisinins in combination with other antimalarials artemisinin-combination therapy, ACT, in order to avoid the development of drug resistance against artemisinin-based therapies.
Severe malaria requires the parenteral administration of antimalarial drugs. Until recently the most used treatment for severe malaria was quinine but artesunate has been shown to be superior to quinine in both children [82] and adults.[83] Treatment of severe malaria also involves supportive measures.
Infection with P. vivax, P. ovale or P. malariae is usually treated on an outpatient basis. Treatment of P. vivax requires both treatment of blood stages (with chloroquine or ACT) as well as clearance of liver forms with primaquine.
Epidemiology


It is estimated that malaria causes 250 million cases of fever and approximately one million deaths annually.[85] The vast majority of cases occur in children under 5 years old;[86] pregnant women are also especially vulnerable. Despite efforts to reduce transmission and increase treatment, there has been little change in which areas are at risk of this disease since 1992.[87] Indeed, if the prevalence of malaria stays on its present upwards course, the death rate could double in the next twenty years.[88] Precise statistics are unknown because many cases occur in rural areas where people do not have access to hospitals or the means to afford health care. As a consequence, the majority of cases are undocumented.[88]
Although co-infection with HIV and malaria does cause increased mortality, this is less of a problem than with HIV/tuberculosis co-infection, due to the two diseases usually attacking different age-ranges, with malaria being most common in the young and active tuberculosis most common in the old.[89] Although HIV/malaria co-infection produces less severe symptoms than the interaction between HIV and TB, HIV and malaria do contribute to each other's spread. This effect comes from malaria increasing viral load and HIV infection increasing a person's susceptibility to malaria infection.[90]
Malaria is presently endemic in a broad band around the equator, in areas of the Americas, many parts of Asia, and much of Africa; however, it is in sub-Saharan Africa where 85– 90% of malaria fatalities occur.[91] The geographic distribution of malaria within large regions is complex, and malaria-afflicted and malaria-free areas are often found close to each other.[92] In drier areas, outbreaks of malaria can be predicted with reasonable accuracy by mapping rainfall.[93] Malaria is more common in rural areas than in cities; this is in contrast to dengue fever where urban areas present the greater risk.[94] For example, several cities in Vietnam, Laos and Cambodia are essentially malaria-free, but the disease is present in many rural regions.[95] By contrast, in Africa malaria is present in both rural and urban areas, though the risk is lower in the larger cities.[96] The global endemic levels of malaria have not been mapped since the 1960s. However, the Wellcome Trust, UK, has funded the Malaria Atlas Project[97] to rectify this, providing a more contemporary and robust means with which to assess current and future malaria disease burden.
History
Malaria has infected humans for over 50,000 years, and Plasmodium may have been a human pathogen for the entire history of the species.[98] Close relatives of the human malaria parasites remain common in chimpanzees.[99] Some new evidence suggests that the most virulent strain of human malaria may have originated in gorillas.[100]
References to the unique periodic fevers of malaria are found throughout recorded history, beginning in 2700 BC in China.[101] Malaria may have contributed to the decline of the Roman Empire,[102] and was so pervasive in Rome that it was known as the "Roman fever".[103] The term malaria originates from Medieval Template:Lang-it — "bad air"; the disease was formerly called ague or marsh fever due to its association with swamps and marshland.[104] Malaria was once common in most of Europe and North America,[105] where it is no longer endemic,[106] though imported cases do occur.[107]
Malaria was the most important health hazard encountered by U.S. troops in the South Pacific during World War II, where about 500,000 men were infected.[108] According to Joseph Patrick Byrne, "Sixty thousand American soldiers died of malaria during the African and South Pacific campaigns."[109]
Prevention
An early effort at malaria prevention occurred in 1896, just before the mosquito malaria link was confirmed in India by a British physician, Ronald Ross. An 1896 Uxbridge malaria outbreak prompted health officer, Dr. Leonard White, to write a report to the Massachusetts State Board of Health, which led to study of mosquito-malaria links, and the first efforts for malaria prevention.[110] Massachusetts State pathologist Theobald Smith, asked that White's son collect mosquito specimens for further analysis, and that citizens 1) add screens to windows, and 2) drain collections of water.[110] Carlos Finlay was also engaged in mosquito related research, and mosquito borne disease theory, in the 1880s in Cuba, basing his work on the study of Yellow Fever.
Discovery of the parasite

"Will cure chills and fever, dyspepsia & c. Will cure bilious fever, liver complaint & c.", c.1881, New York

Scientific studies on malaria made their first significant advance in 1880, when a French army doctor working in the military hospital of Constantine in Algeria named Charles Louis Alphonse Laveran observed parasites for the first time, inside the red blood cells of people suffering from malaria. He, therefore, proposed that malaria is caused by this organism, the first time a protist was identified as causing disease.[111] For this and later discoveries, he was awarded the 1907 Nobel Prize for Physiology or Medicine. The malarial parasite was called Plasmodium by the Italian scientists Ettore Marchiafava and Angelo Celli.[112]
Discovery of mosquito transmission
A year later, Carlos Finlay, a Cuban doctor treating patients with yellow fever in Havana, provided strong evidence that mosquitoes were transmitting disease to and from humans.[113] This work followed earlier suggestions by Josiah C. Nott,[114] and work by Patrick Manson on the transmission of filariasis.[115]
It was Britain's Sir Ronald Ross, working in the Presidency General Hospital in Calcutta, who finally proved in 1898 that malaria is transmitted by mosquitoes. He did this by showing that certain mosquito species transmit malaria to birds. He isolated malaria parasites from the salivary glands of mosquitoes that had fed on infected birds.[116] For this work, Ross received the 1902 Nobel Prize in Medicine. After resigning from the Indian Medical Service, Ross worked at the newly established Liverpool School of Tropical Medicine and directed malaria-control efforts in Egypt, Panama, Greece and Mauritius.[117] The findings of Finlay and Ross were later confirmed by a medical board headed by Walter Reed in 1900. Its recommendations were implemented by William C. Gorgas in the health measures undertaken during construction of the Panama Canal. This public-health work saved the lives of thousands of workers and helped develop the methods used in future public-health campaigns against the disease.
The liver stage
Shortt and Garnham discovered the pre-erythrocytic liver stage, first in the primate parasite P. cynomolgi and subsequently the human malarias P. vivax [118] and P. falciparum.[119] Further work confirmed the transformation of sporozoites from mosquitoes into liver forms, essentially completing documentation of the lifecycle.[120]
In vitro culture
The first successful continuous in vitro malaria culture was established in 1976 by William Trager and James B. Jensen, which facilitated research into the molecular biology of the parasite and the development of new drugs.[121][122]
History of treatment
The first effective treatment for malaria came from the bark of cinchona tree, which contains quinine. This tree grows on the slopes of the Andes, mainly in Peru. The indigenous peoples of Peru made a tincture of cinchona to control malaria. The Jesuits noted the efficacy of the practice and introduced the treatment to Europe during the 1640s, where it was rapidly accepted.[123] It was not until 1820 that the active ingredient, quinine, was extracted from the bark, isolated and named by the French chemists Pierre Joseph Pelletier and Joseph Bienaimé Caventou.[124] In the 20th century, chloroquine replaced quinine as treatment of both uncomplicated and severe falciparum malaria until resistance supervened. Artemisinins, discovered by Chinese scientists in the 1970s, are now recommended treatment for falciparum malaria, administered in combination with other antimalarials as well as in severe disease.
Society and culture
Malaria is not just a disease commonly associated with poverty but also a cause of poverty and a major hindrance to economic development. Tropical regions are affected most, however malaria’s furthest extent reaches into some temperate zones with extreme seasonal changes. The disease has been associated with major negative economic effects on regions where it is widespread. During the late 19th and early 20th centuries, it was a major factor in the slow economic development of the American southern states.[125] A comparison of average per capita GDP in 1995, adjusted for parity of purchasing power, between countries with malaria and countries without malaria gives a fivefold difference ($1,526 USD versus $8,268 USD). In countries where malaria is common, average per capita GDP has risen (between 1965 and 1990) only 0.4% per year, compared to 2.4% per year in other countries.[126]
Poverty is both cause and effect, however, since the poor do not have the financial capacities to prevent or treat the disease. The lowest income group in Malawi carries (1994) the burden of having 32% of their annual income used on this disease compared with the 4% of household incomes from low-to-high groups.[127] In its entirety, the economic impact of malaria has been estimated to cost Africa $12 billion USD every year. The economic impact includes costs of health care, working days lost due to sickness, days lost in education, decreased productivity due to brain damage from cerebral malaria, and loss of investment and tourism.[86] In some countries with a heavy malaria burden, the disease may account for as much as 40% of public health expenditure, 30–50% of inpatient admissions, and up to 50% of outpatient visits.[128]
A study on the effect of malaria on IQ in a sample of Mexicans found that exposure during the birth year to malaria eradication was associated with increases in IQ. It also increased the probability of employment in a skilled occupation. The author suggests that this may be one explanation for the Flynn effect and that this may be an important explanation for the link between national malaria burden and economic development.[129] A literature review of 44 papers states that cognitive abilities and school performance were shown to be impaired in sub-groups of patients (with either cerebral malaria or uncomplicated malaria) when compared with healthy controls. Studies comparing cognitive functions before and after treatment for acute malarial illness continued to show significantly impaired school performance and cognitive abilities even after recovery. Malaria prophylaxis was shown to improve cognitive function and school performance in clinical trials when compared to placebo groups.[130]
Counterfeit drugs
Sophisticated counterfeits have been found in several Asian countries such as Cambodia,[131] China,[132] Indonesia, Laos, Thailand, Vietnam and are an important cause of avoidable death in those countries.[133] WHO have said that studies indicate that up to 40% of artesunate based malaria medications are counterfeit, especially in the Greater Mekong region and have established a rapid alert system to enable information about counterfeit drugs to be rapidly reported to the relevant authorities in participating countries.[134] There is no reliable way for doctors or lay people to detect counterfeit drugs without help from a laboratory. Companies are attempting to combat the persistence of counterfeit drugs by using new technology to provide security from source to distribution.
War
Throughout history, the contraction of malaria (via natural outbreaks as well as via infliction of the disease as a biological warfare agent) has played a prominent role in the fortunes of government rulers, nation-states, military personnel, and military actions. "Malaria Site: History of Malaria During Wars" addresses the devastating impact of malaria in numerous well-known conflicts, beginning in June 323 B.C. That site's authors note: "Many great warriors succumbed to malaria after returning from the warfront and advance of armies into continents was prevented by malaria. In many conflicts, more troops were killed by malaria than in combat."[135] The Centers for Disease Control ("CDC") traces the history of malaria and its impacts farther back, to 2700 BCE.[136]
In 1910, Nobel Prize in Medicine-winner Ronald Ross (himself a malaria survivor), published a book titled The Prevention of Malaria that included the chapter: "The Prevention of Malaria in War." The chapter's author, Colonel C. H. Melville, Professor of Hygiene at Royal Army Medical College in London, addressed the prominent role that malaria has historically played during wars and advised: "A specially selected medical officer should be placed in charge of these operations with executive and disciplinary powers [...]."
Significant financial investments have been made to fund procure existing and create new anti-malarial agents. During World War I and World War II, the supplies of the natural anti-malaria drugs, cinchona bark and quinine, proved to be inadequate to supply military personnel and substantial funding was funnelled into research and development of other drugs and vaccines. American military organizations conducting such research initiatives include the Navy Medical Research Center, Walter Reed Army Institute of Research, and the U.S. Army Medical Research Institute of Infectious Diseases of the US Armed Forces.[135]
Additionally, initiatives have been founded such as Malaria Control in War Areas (MCWA), established in 1942, and its successor, the Communicable Disease Center (now known as the Centers for Disease Control) established in 1946. According to the CDC, MCWA "was established to control malaria around military training bases in the southern United States and its territories, where malaria was still problematic" and, during these activities, to "train state and local health department officials in malaria control techniques and strategies." The CDC's Malaria Division continued that mission, successfully reducing malaria in the United States, after which the organization expanded its focus to include "prevention, surveillance, and technical support both domestically and internationally."[136]
Popular culture
- Joseph Conrad, author of the novella Heart of Darkness (published in serial form in 1899 and as a novella in 1902), contracted malaria while on a four-month steamboat voyage along the Congo River.[137] One character in the book, an ivory trader named Kurtz, dies from the disease[137] and the novella's narrator, Charles Marlow, is weakened by it.
- In making the movie The African Queen (1951), most of the crew and the leading lady, Katharine Hepburn, endured malaria.[138][139][140]
- In the movie Apocalypse Now (1979), Captain Benjamin L. Willard is a special operations veteran stationed in Southeast Asia during the Vietnam war. Willard is assigned a classified mission to assassinate rogue soldier, Colonel Walter E. Kurtz, who has gone insane and is commanding a legion of his own Montagnard troops in neutral Cambodia. Upon finding Kurtz's location, Willard narrates: "It smelled like slow death in there, malaria, and nightmares. This was the end of the river, all right."[141]
- In Barbara Kingsolver's novel, The Poisonwood Bible (1998), Ruth May – the youngest daughter of an American Christian missionary who brings his family to live in the Belgian Congo of the late 1950s – stops taking her quinine tablets and contracts malaria.[142]
Research
With the onset of drug resistant Plasmodium parasites, new strategies are required to combat the widespread disease. One such approach lies in the introduction of synthetic pyridoxal-amino acid adducts, which are channelled into the parasite. Thus, trapped upon phosphorylation by plasmodial PdxK (pyridoxine/pyridoxal kinase), the proliferation of Plasmodium parasites is effectively hindered by a novel compound, PT3, a cyclic pyridoxyl-tryptophan methyl ester, without harming human cells.[143]
References
- ^ Fong YL, Cadigan FC, Coatney GR (1971). "A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia". Trans. R. Soc. Trop. Med. Hyg. 65 (6): 839–40. doi:10.1016/0035-9203(71)90103-9. PMID 5003320.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Singh B, Kim Sung L, Matusop A; et al. (2004). "A large focus of naturally acquired Plasmodium knowlesi infections in human beings". Lancet. 363 (9414): 1017–24. doi:10.1016/S0140-6736(04)15836-4. PMID 15051281.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kilama W, Ntoumi F (2009). "Malaria: a research agenda for the eradication era". Lancet. 374 (9700): 1480–2. doi:10.1016/S0140-6736(09)61884-5. PMID 19880004.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dondorp AM, Day NP (2007). "The treatment of severe malaria". Trans. R. Soc. Trop. Med. Hyg. 101 (7): 633–4. doi:10.1016/j.trstmh.2007.03.011. PMID 17434195.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dondorp AM, Fanello CI, Hendriksen IC; et al. (2010). "Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial". Lancet. 376 (9753): 1647–57. doi:10.1016/S0140-6736(10)61924-1. PMC 3033534. PMID 21062666.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wellems TE (2002). "Plasmodium chloroquine resistance and the search for a replacement antimalarial drug". Science. 298 (5591): 124–6. doi:10.1126/science.1078167. PMID 12364789.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Phillips, Nicky (September 26, 2010). "Gorillas in midst of malaria mystery". Sydney Morning Hearld. Retrieved September 28, 2010.
- ^ Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI (2005). "The global distribution of clinical episodes of Plasmodium falciparum malaria". Nature. 434 (7030): 214–7. doi:10.1038/nature03342. PMID 15759000.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Malaria: Disease Impacts and Long-Run Income Differences" (PDF). Institute for the Study of Labor. Retrieved 2008-12-10.
- ^ http://www.talksy.com/28786/stunning-data-point-malaria-parasite-responsible-for-half-of-all-human-deaths-since-stone-age. Retrieved 2008-12-10.
{{cite web}}: Missing or empty|title=(help) - ^ WebMD > Malaria symptoms Last Updated: May 16, 2007
- ^ Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME (November 1, 2006). "Malarial retinopathy: a newly established diagnostic sign in severe malaria". Am. J. Trop. Med. Hyg. 75 (5): 790–7. PMC 2367432. PMID 17123967.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Malaria life cycle & pathogenesis. Malaria in Armenia. Retrieved October 31, 2006.
- ^ Idro, R. "Decorticate, decerebrate and opisthotonic posturing and seizures in Kenyan children with cerebral malaria". Malaria Journal. 4 (57): 57. doi:10.1186/1475-2875-4-57. PMC 1326205. PMID 16336645.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Boivin MJ (2002). "Effects of early cerebral malaria on cognitive ability in Senegalese children". J Dev Behav Pediatr. 23 (5): 353–64. PMID 12394524.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Holding PA, Snow RW (2001). "Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence". Am. J. Trop. Med. Hyg. 64 (1–2 Suppl): 68–75. PMID 11425179. Scholar search
- ^ Maude RJ, Hassan MU, Beare NAV (June 1, 2009). "Severe retinal whitening in an adult with cerebral malaria". Am J Trop Med Hyg. 80 (6): 881. PMC 2843443. PMID 19478242.
{{cite journal}}: Unknown parameter|unused_data=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Beare NAV, Taylor TE, Harding SP, Lewallen S, Molyneux ME (2006). "Malarial retinopathy: a newly established diagnostic sign in severe malaria". Am J Trop Med Hyg. 75 (5): 790–797. PMC 2367432. PMID 17123967.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Trampuz A, Jereb M, Muzlovic I, Prabhu R (2003). "Clinical review: Severe malaria". Crit Care. 7 (4): 315–23. doi:10.1186/cc2183. PMC 270697. PMID 12930555.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Kain K, Harrington M, Tennyson S, Keystone J (1998). "Imported malaria: prospective analysis of problems in diagnosis and management". Clin Infect Dis. 27 (1): 142–9. doi:10.1086/514616. PMID 9675468.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mockenhaupt F, Ehrhardt S, Burkhardt J, Bosomtwe S, Laryea S, Anemana S, Otchwemah R, Cramer J, Dietz E, Gellert S, Bienzle U (2004). "Manifestation and outcome of severe malaria in children in northern Ghana". Am J Trop Med Hyg. 71 (2): 167–72. PMID 15306705.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carter JA, Ross AJ, Neville BG, Obiero E, Katana K, Mung'ala-Odera V, Lees JA, Newton CR (2005). "Developmental impairments following severe falciparum malaria in children". Trop Med Int Health. 10 (1): 3–10. doi:10.1111/j.1365-3156.2004.01345.x. PMID 15655008.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mueller I, Zimmerman PA, Reeder JC (2007). "Plasmodium malariae and Plasmodium ovale—the "bashful" malaria parasites". Trends Parasitol. 23 (6): 278–83. doi:10.1016/j.pt.2007.04.009. PMID 17459775.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Singh B, Kim Sung L, Matusop A; et al. (2004). "A large focus of naturally acquired Plasmodium knowlesi infections in human beings". Lancet. 363 (9414): 1017–24. doi:10.1016/S0140-6736(04)15836-4. PMID 15051281.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mendis K, Sina B, Marchesini P, Carter R (2001). "The neglected burden of Plasmodium vivax malaria" (PDF). Am J Trop Med Hyg. 64 (1–2 Suppl): 97–106. PMID 11425182.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Escalante A, Ayala F (1994). "Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences". Proc Natl Acad Sci USA. 91 (24): 11373–7. doi:10.1073/pnas.91.24.11373. PMC 45233. PMID 7972067.
- ^ Garnham, PCC (1966). Malaria parasites and other haemosporidia. Oxford: Blackwell Scientific Publications. ISBN 186983500X.
- ^ Collins, WE; Barnwell, JW (2009). "Plasmodium knowlesi: Finally being recognized". J Infect Dis. 199 (8): 1107–1108. doi:10.1086/597415. PMID 19284287.
{{cite journal}}: More than one of|author1=and|last1=specified (help); More than one of|author2=and|last2=specified (help) - ^ Köhler, Sabine; Delwiche, CF; Denny, PW; Tilney, LG; Webster, P; Wilson, RJ; Palmer, JD; Roos, DS (1997). "A Plastid of Probable Green Algal Origin in Apicomplexan Parasites". Science. 275 (5305): 1485–1489. doi:10.1126/science.275.5305.1485. PMID 9045615.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help); Unknown parameter|month=ignored (help) - ^ Gardner, Malcom; Tettelin, H; Carucci, DJ; Cummings, LM; Aravind, L; Koonin, EV; Shallom, S; Mason, T; Yu, K (1998). "Chromosome 2 Sequence of the Human Malaria Parasite Plasmodium falciparum". Science. 282 (5391): 1126–1132. doi:10.1126/science.282.5391.1126. PMID 9804551.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help); Unknown parameter|month=ignored (help) - ^ Foth, Bernado; Ralph, SA; Tonkin, CJ; Struck, NS; Fraunholz, M; Roos, DS; Cowman, AF; McFadden, GI (2003). "Dissecting Apicoplast Targeting in the Malaria Parasite Plasmodium falciparum". Science. 299 (5607): 705–708. doi:10.1126/science.1078599. PMID 12560551.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help); Unknown parameter|month=ignored (help) - ^ Talman A, Domarle O, McKenzie F, Ariey F, Robert V (2004). "Gametocytogenesis: the puberty of Plasmodium falciparum". Malar J. 3: 24. doi:10.1186/1475-2875-3-24. PMC 497046. PMID 15253774.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Mosquitoe Facts
- ^ Marcucci C, Madjdpour C, Spahn D (2004). "Allogeneic blood transfusions: benefit, risks and clinical indications in countries with a low or high human development index". Br Med Bull. 70: 15–28. doi:10.1093/bmb/ldh023. PMID 15339855.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Adak T, Sharma V, Orlov V (1998). "Studies on the Plasmodium vivax relapse pattern in Delhi, India". Am J Trop Med Hyg. 59 (1): 175–9. PMID 9684649.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bledsoe GH (2005). "Malaria primer for clinicians in the United States". South. Med. J. 98 (12): 1197–204, quiz 1205, 1230. doi:10.1097/01.smj.0000189904.50838.eb. PMID 16440920.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Sturm A,
Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT (2006). "Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids". Science. 313 (5791): 1287–1490. doi:10.1126/science.1129720. PMID 16888102.
{{cite journal}}: line feed character in|author=at position 9 (help)CS1 maint: multiple names: authors list (link) - ^ Cogswell FB (1992). "The hypnozoite and relapse in primate malaria". Clin. Microbiol. Rev. 5 (1): 26–35. PMC 358221. PMID 1735093.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Chen Q, Schlichtherle M, Wahlgren M (2000). "Molecular aspects of severe malaria". Clin. Microbiol. Rev. 13 (3): 439–50. doi:10.1128/CMR.13.3.439-450.2000. PMC 88942. PMID 10885986.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Adams S, Brown H, Turner G (2002). "Breaking down the blood-brain barrier: signaling a path to cerebral malaria?". Trends Parasitol. 18 (8): 360–6. doi:10.1016/S1471-4922(02)02353-X. PMID 12377286.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G (2000). "Effect of pregnancy on exposure to malaria mosquitoes". Lancet. 355 (9219): 1972. doi:10.1016/S0140-6736(00)02334-5. PMID 10859048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ van Geertruyden J, Thomas F, Erhart A, D'Alessandro U (August 1, 2004). "The contribution of malaria in pregnancy to perinatal mortality". Am J Trop Med Hyg. 71 (2 Suppl): 35–40. PMID 15331817.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rodriguez-Morales AJ, Sanchez E, Vargas M, Piccolo C, Colina R, Arria M, Franco-Paredes C (2006). "Pregnancy outcomes associated with Plasmodium vivax malaria in northeastern Venezuela". Am J Trop Med Hyg. 74 (5): 755–757. PMID 16687675.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kwiatkowski DP (2005). "How malaria has affected the human genome and what human genetics can teach us about malaria". Am J Hum Genet. 77 (2): 171–92. doi:10.1086/432519. PMC 1224522. PMID 16001361.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Krafts K, Hempelmann E, Oleksyn B (2011). "The color purple: from royalty to laboratory, with apologies to Malachowski". Biotech Histochem. 86: 7–35. doi:10.3109/10520295.2010.515490. PMID 21235291.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sutherland, CJ; Hallett, R (2009). "Detecting malaria parasites outside the blood". J Infect Dis. 199 (11): 1561–1563. doi:10.1086/598857. PMID 19432543.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ Redd S, Kazembe P, Luby S, Nwanyanwu O, Hightower A, Ziba C, Wirima J, Chitsulo L, Franco C, Olivar M (2006). "Clinical algorithm for treatment of Plasmodium falciparum malaria in children". Lancet. 347 (8996): 80. doi:10.1016/S0140-6736(96)90404-3. PMID 8551881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Warhurst DC, Williams JE (1996). "Laboratory diagnosis of malaria". J Clin Pathol. 49 (7): 533–38. doi:10.1136/jcp.49.7.533. PMC 500564. PMID 8813948.
- ^ McCutchan, Thomas F. (2008). "Use of Malaria Rapid Diagnostic Test to Identify Plasmodium knowlesi Infection". Emerging Infectious Disease. 14 (11). Centers for Disease Control: 1750–1752. doi:10.3201/eid1411.080840. PMC 2630758. PMID 18976561.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Pattanasin S, Proux S, Chompasuk D, Luwiradaj K, Jacquier P, Looareesuwan S, Nosten F (2003). "Evaluation of a new Plasmodium lactate dehydrogenase assay (OptiMAL-IT) for the detection of malaria". Transact Royal Soc Trop Med. 97 (6): 672–4. doi:10.1016/S0035-9203(03)80100-1. PMID 16117960.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ling IT., Cooksley S., Bates PA., Hempelmann E., Wilson RJM. (1986). "Antibodies to the glutamate dehydrogenase of Plasmodium falciparum". Parasitology. 92, : 313–24. doi:10.1017/S0031182000064088. PMID 3086819.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Mens, PF; Schoone, GJ; Kager, PA; Schallig, HD (2006). "Detection and identification of human Plasmodium species with real-time quantitative nucleic acid sequence-based amplification". Malaria Journal. 5 (80): 80. doi:10.1186/1475-2875-5-80. PMC 1592503. PMID 17018138.
{{cite journal}}: More than one of|author=and|last1=specified (help)CS1 maint: unflagged free DOI (link) - ^ Redd S, Kazembe P, Luby S, Nwanyanwu O, Hightower A, Ziba C, Wirima J, Chitsulo L, Franco C, Olivar M (2006). "Clinical algorithm for treatment of Plasmodium falciparum malaria in children". Lancet. 347 (8996): 80. doi:10.1016/S0140-6736(96)90404-3. PMID 8551881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME (2006). "Malarial retinopathy: a newly established diagnostic sign in severe malaria". Am. J. Trop. Med. Hyg. 75 (5): 790–7. PMC 2367432. PMID 17123967.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Medical News Today, 2007". Medicalnewstoday.com. Retrieved 2010-08-24.
- ^ Snow, Robert W. (2008). "International Funding for Malaria Control in Relation to Populations at Risk of Stable Plasmodium falciparum Transmission". PLoS Medicine. 5 (7). Public Library of Science: e142. doi:10.1371/journal.pmed.0050142. PMC 2488181. PMID 18651785.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Barat L (2006). "Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam". Am J Trop Med Hyg. 74 (1): 12–6. PMID 16407339.
- ^ Jacquerioz FA, Croft AM (2009). "Drugs for preventing malaria in travellers". Cochrane Database Syst Rev (4): CD006491. doi:10.1002/14651858.CD006491.pub2. PMID 19821371.
- ^ Roestenberg M, McCall M, Hopman J; et al. (2009). "Protection against a malaria challenge by sporozoite inoculation". N. Engl. J. Med. 361 (5): 468–77. doi:10.1056/NEJMoa0805832. PMID 19641203.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b http://www.cdc.gov/malaria/history/eradication_us.htm Centers for Disease Control. Eradication of Malaria in the United States (1947–1951) 2004.
- ^ Killeen G, Fillinger U, Kiche I, Gouagna L, Knols B (2002). "Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa?". Lancet Infect Dis. 2 (10): e192. doi:10.1016/S1473-3099(02)00397-3. PMID 12383612.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Imperial College, London, "Scientists create first transgenic malaria mosquito", 2000-06-22.
- ^ Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M (2002). "Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite". Nature. 417 (6887): 387–8. doi:10.1038/417452a. PMID 12024215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Knols et al., 2007
- ^ Robert Guth (2009-03-14). "Rocket Scientists Shoot Down Mosquitoes With Lasers". WSJ.com. Retrieved 8 July 2009.
- ^ Tia E, Akogbeto M, Koffi A; et al. (2006). "[Pyrethroid and DDT resistance of Anopheles gambiae s.s. (Diptera: Culicidae) in five agricultural ecosystems from Côte-d'Ivoire]". Bulletin de la Société de pathologie exotique (1990) (in French). 99 (4): 278–82. PMID 17111979.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Indoor Residual Spraying: Use of Indoor Residual Spraying for Scaling Up Global Malaria Control and Elimination. World Health Organization, 2006.
- ^ van den Berg, Henk (2009). "Global Status of DDT and Its Alternatives for Use in Vector Control to Prevent Disease". Environmental Health Perspectives. 117 (11): 1656–1663. doi:10.1289/ehp.0900785. ISSN 0091-6765. PMC 2801202. PMID 20049114.
- ^ Rosenberg, Tina (April 11, 2004). "What the world needs now is DDT". New York Times. Retrieved 2008-11-03.
- ^ Pates, H & Curtis, C.:"Mosquito behaviour and vector control", page 53-70. Annual Review on Entomology, 50, 2005.
- ^ Bachou H, Tylleskär T, Kaddu-Mulindwa DH, Tumwine JK (2006). "Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda". BMC Infect. Dis. 6: 160. doi:10.1186/1471-2334-6-160. PMC 1660577. PMID 17090299.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Färnert, A; Williams, TN; Mwangi, TW; Ehlin, A; Fegan, G; Macharia, A; Lowe, BS; Montgomery, SM; Marsh, K (2009). "Transmission‐dependent tolerance to multiclonal Plasmodium falciparum infection". J Infect Dis. 200 (7): 1166–1175. doi:10.1086/605652. PMC 2741682. PMID 19702508.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ Nussenzweig R, Vanderberg J, Most H, Orton C (1967). "Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei". Nature. 216 (5111): 160–2. doi:10.1038/216160a0. PMID 6057225.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hoffman SL, Goh LM, Luke TC; et al. (2002). "Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites". J. Infect. Dis. 185 (8): 1155–64. doi:10.1086/339409. PMID 11930326.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "What is Malariacontrol.net". AFRICA@home. Retrieved 2007-03-11.
- ^ Águas R, White LJ, Snow RW, Gomes MG (2008). "Prospects for malaria eradication in sub-Saharan Africa". PLoS ONE. 3 (3): e1767. doi:10.1371/journal.pone.0001767. PMC 2262141. PMID 18335042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Laser pest control physics.org, May 2010
- ^ Kare, Jordin. "Backyard Star Wars: Build your own photonic fence to zap mosquitoes midflight". IEEE Spectrum (2010 May).
- ^ Corby-Harris V, Drexler A, Watkins de Jong L, Antonova Y, Pakpour N; et al. (2010). "Activation of Akt Signaling Reduces the Prevalence and Intensity of Malaria Parasite Infection and Lifespan in Anopheles stephensi Mosquitoes". PLoS Pathog. 6 (7): e1001003. doi:10.1371/journal.ppat.1001003. PMC 2904800. PMID 20664791.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Gill, Victoria (16 July 2010). "Malaria-proof mosquito engineered". BBC News Online.
- ^ If I get malaria, will I have it for the rest of my life? CDC publication, Accessed 14 November 2006.
- ^ Dondorp AM, Fanello CI, Hendriksen IC; et al. (2010). "Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial". Lancet. 376 (9753): 1647–57. doi:10.1016/S0140-6736(10)61924-1. PMC 3033534. PMID 21062666.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dondorp A, Nosten F, Stepniewska K, Day N, White N (2005). "Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial". Lancet. 366 (9487): 717–25. doi:10.1016/S0140-6736(05)67176-0. PMID 16125588.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Malaria". US Centers for Disease Control and Prevention. 2003. Retrieved 2008-07-20.
- ^ 2005 WHO World Malaria Report 2008
- ^ a b Greenwood BM, Bojang K, Whitty CJ, Targett GA (2005). "Malaria". Lancet. 365 (9469): 1487–1498. doi:10.1016/S0140-6736(05)66420-3. PMID 15850634.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hay S, Guerra C, Tatem A, Noor A, Snow R (2004). "The global distribution and population at risk of malaria: past, present, and future". Lancet Infect Dis. 4 (6): 327–36. doi:10.1016/S1473-3099(04)01043-6. PMID 15172341.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Breman J (January 1, 2001). "The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden". Am J Trop Med Hyg. 64 (1–2 Suppl): 1–11. PMID 11425172.
- ^ Korenromp E, Williams B, de Vlas S, Gouws E, Gilks C, Ghys P, Nahlen B (2005). "Malaria attributable to the HIV-1 epidemic, sub-Saharan Africa". Emerg Infect Dis. 11 (9): 1410–9. PMID 16229771.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Abu-Raddad L, Patnaik P, Kublin J (2006). "Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa". Science. 314 (5805): 1603–6. doi:10.1126/science.1132338. PMID 17158329.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Layne SP. "Principles of Infectious Disease Epidemiology /" (PDF). EPI 220. UCLA Department of Epidemiology. Archived from the original (PDF) on 2006-02-20. Retrieved 2007-06-15.
- ^ Greenwood B, Mutabingwa T (2002). "Malaria in 2002". Nature. 415 (6872): 670–2. doi:10.1038/415670a. PMID 11832954.
- ^ Grover-Kopec E, Kawano M, Klaver R, Blumenthal B, Ceccato P, Connor S (2005). "An online operational rainfall-monitoring resource for epidemic malaria early warning systems in Africa". Malar J. 4: 6. doi:10.1186/1475-2875-4-6. PMC 548290. PMID 15663795.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Van Benthem B, Vanwambeke S, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, Lambin E, Somboon P (February 1, 2005). "Spatial patterns of and risk factors for seropositivity for dengue infection". Am J Trop Med Hyg. 72 (2): 201–8. PMID 15741558.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Trung H, Van Bortel W, Sochantha T, Keokenchanh K, Quang N, Cong L, Coosemans M (2004). "Malaria transmission and major malaria vectors in different geographical areas of Southeast Asia". Trop Med Int Health. 9 (2): e473. doi:10.1046/j.1365-3156.2003.01179.x. PMID 15040560.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Keiser J, Utzinger J, Caldas de Castro M, Smith T, Tanner M, Singer B (August 1, 2004). "Urbanization in sub-saharan Africa and implication for malaria control". Am J Trop Med Hyg. 71 (2 Suppl): 118–27. PMID 15331827.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hay SI, Snow RW (2006). "The Malaria Atlas Project: Developing Global Maps of Malaria Risk". PLoS Medicine. 3 (12): e473. doi:10.1371/journal.pmed.0030473. PMC 1762059. PMID 17147467.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Joy D, Feng X, Mu J; et al. (2003). "Early origin and recent expansion of Plasmodium falciparum". Science. 300 (5617): 318–21. doi:10.1126/science.1081449. PMID 12690197.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Escalante A, Freeland D, Collins W, Lal A (1998). "The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome". Proc Natl Acad Sci USA. 95 (14): 8124–9. doi:10.1073/pnas.95.14.8124. PMC 20940. PMID 9653151.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Liu, W (2010). "Origin of the human malaria parasite Plasmodium falciparum in gorillas". Nature. 467.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cox F (2002). "History of human parasitology". Clin Microbiol Rev. 15 (4): 595–612. doi:10.1128/CMR.15.4.595-612.2002. PMC 126866. PMID 12364371.
- ^ DNA clues to malaria in ancient Rome. BBC News. February 20, 2001.
- ^ "Malaria and Rome". Robert Sallares. ABC.net.au. January 29, 2003.
- ^ From Shakespeare to Defoe: Malaria in England in the Little Ice Age. Paul Reiter. Centers for Disease Control and Prevention, San Juan, Puerto Rico.
- ^ "Medicine and society in early modern Europe". Mary Lindemann (1999). Cambridge University Press. p.62. ISBN 0521423546
- ^ Vector- and Rodent-Borne Diseases in Europe and North America. Norman G. Gratz. World Health Organization, Geneva.
- ^ James L. A. Webb, Jr., Humanity’s Burden: A Global History of Malaria (Cambridge University Press, 2009)
- ^ "Armies of pestilence: the effects of pandemics on history". James Clarke & Co. (2004). p.102. ISBN 022717240X
- ^ "Encyclopedia of Pestilence, Pandemics, and Plagues: A-M". Joseph Patrick Byrne (2008). p.383. ISBN 0313341028
- ^ a b "A History of Mosquitoes in Massachusetts, by Curtis R. Best". Northeast Mosquito Control Association. Retrieved 2008-03-31.
- ^ "Biography of Alphonse Laveran". The Nobel Foundation. Retrieved 2007-06-15. ] Nobel foundation. Accessed 25 October 2006
- ^ "Ettore Marchiafava". Retrieved 2007-06-15.
- ^ Tan SY, Sung H (2008). "Carlos Juan Finlay (1833–1915): of mosquitoes and yellow fever" (PDF). Singapore Med J. 49 (5): 370–1. PMID 18465043.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chernin E (1983). "Josiah Clark Nott, insects, and yellow fever". Bull N Y Acad Med. 59 (9): 790–802. PMC 1911699. PMID 6140039.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chernin E (1977). "Patrick Manson (1844–1922) and the transmission of filariasis". Am. J. Trop. Med. Hyg. 26 (5 Pt 2 Suppl): 1065–70. PMID 20786.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Biography of Ronald Ross". The Nobel Foundation. Retrieved 2007-06-15.
- ^ "Ross and the Discovery that Mosquitoes Transmit Malaria Parasites". CDC Malaria website. Archived from the original on June 2, 2007. Retrieved 2007-06-15.
- ^ SHORTT HE, GARNHAM PC (1948). "The pre-erythrocytic stage of human malaria, Plasmodium vivax". Br Med J. 1 (4550): 547. PMC 2090089. PMID 18909485.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ SHORTT HE, FAIRLEY NH, COVELL G, SHUTE PG, GARNHAM PC (1951). "The pre-erythrocytic stage of Plasmodium falciparum". Trans. R. Soc. Trop. Med. Hyg. 44 (4): 405–19. PMID 14817818.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Meis JF, Verhave JP, Jap PH, Sinden RE, Meuwissen JH (1983). "Malaria parasites--discovery of the early liver form". Nature. 302 (5907): 424–6. PMID 6339945.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Trager W, Jensen JB (1976). "Human malaria parasites in continuous culture". Science. 193 (4254): 673–675. doi:10.1126/science.781840. PMID 781840.
- ^ Schuster FL (2002). "Cultivation of plasmodium spp". Clin Microbiol Rev. 15 (3): 355–364. doi:10.1128/CMR.15.3.355-364.2002. PMC 118084. PMID 12097244.
- ^ Kaufman T, Rúveda E (2005). "The quest for quinine: those who won the battles and those who won the war". Angew Chem Int Ed Engl. 44 (6): 854–85. doi:10.1002/anie.200400663. PMID 15669029.
- ^ Kyle R, Shampe M (1974). "Discoverers of quinine". JAMA. 229 (4): e320. doi:10.1001/jama.229.4.462. PMID 4600403.
- ^ Humphreys, M (2001). Malaria: Poverty, Race, and Public Health in the United States. Johns Hopkins University Press. p. 256. ISBN 0-8018-6637-5.
- ^ Sachs J, Malaney P (2002). "The economic and social burden of malaria". Nature. 415 (6872): 680–5. doi:10.1038/415680a. PMID 11832956.
- ^ Ettling M, McFarland DA, Schultz LJ, Chitsulo L (1994). "Economic impact of malaria in Malawian households". Trop. Med. Parasitol. 45 (1): 74–9. PMID 8066390.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Roll Back Malaria WHO partnership. "Economic costs of malaria" (PDF). WHO. Retrieved 2009-09-18.
- ^ Venkataramani, Atheendar, Early Life Exposure to Malaria and Cognition and Skills in Adulthood: Evidence from Mexico (September 18, 2010). Available at SSRN: http://ssrn.com/abstract=1679164
- ^ The ‘hidden’ burden of malaria: cognitive impairment following infection. Sumadhya D Fernando, Chaturaka Rodrigo, and Senaka Rajapakse. Malaria Journal 2010, 9:366. http://preview.malariajournal.com/content/pdf/1475-2875-9-366.pdf
- ^ Lon CT, Tsuyuoka R, Phanouvong S; et al. (2006). "Counterfeit and substandard antimalarial drugs in Cambodia". Trans R Soc Trop Med Hyg. 100 (11): 1019–24. doi:10.1016/j.trstmh.2006.01.003. PMID 16765399.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ U. S. Pharmacopeia (2004). "Fake antimalarials found in Yunan province, China" (PDF). Retrieved 2006-10-06.
- ^ Newton PN Green MD, Fernández FM, Day NPJ, White NJ. (2006). "Counterfeit anti-infective drugs". Lancet Infect Dis. 6 (9): 602–13. doi:10.1016/S1473-3099(06)70581-3. PMID 16931411.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jane Parry (2005). "WHO combats counterfeit malaria drugs in Asia". Retrieved 2008-07-19.
- ^ a b http://www.malariasite.com/malaria/history_wars.htm
- ^ a b "History | CDC Malaria". Cdc.gov. 2004-04-23. Retrieved 2010-08-24.
- ^ a b Heart of Darkness and Selected Short ... – Google Books. Books.google.com. 2003-09-01. ISBN 9781593080211. Retrieved 2010-08-24.
- ^ "Film Notes -The African Queen". Albany.edu. Retrieved 2010-08-24.
- ^ Lumenick, Lou (2010-03-21). "Bogie-Hepburn classic, 'The African Queen' finally lands on DVD". NYPOST.com. Retrieved 2010-08-24.
- ^ "The African Queen – Bogart, Hepburn, and a case of the 'jungle jeebies' – Features, Films". London: The Independent. 2010-05-07. Retrieved 2010-08-24.
- ^ "Apocalypse Now (1979)". Filmsite.org. Retrieved 2010-08-24.
- ^ "The Poisonwood Bible – Summary". GradeSaver. 2008-05-01. Retrieved 2010-08-24.
- ^ Müller IB, Wu F, Bergmann B; et al. (2009). "Poisoning pyridoxal 5-phosphate-dependent enzymes: a new strategy to target the malaria parasite Plasmodium falciparum". PLoS ONE. 4 (2): e4406. doi:10.1371/journal.pone.0004406. PMC 2634962. PMID 19197387.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)
External links
- WHO site on malaria
- Global Malaria action Plan
- Malaria Atlas Project
- Medicines for Malaria Venture (MMV)
- World Malaria Report 2005
- Doctors Without Borders/Médecins Sans Frontières – Malaria information pages
- Medline Plus – Malaria
- Who/TDR Malaria Database
- Anti malaria and sustainable development
- Worldwide Antimalarial Resistance Network (WWARN)
- Template:Dmoz
Template:Link GA Template:Link FA Template:Link FA Template:Link FA
