Selenium dioxide
| |

| |

| |
| Names | |
|---|---|
| Other names
Selenium(IV) oxide
Selenous anhydride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.358 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3283 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SeO2 | |
| Molar mass | 110.96 g/mol |
| Appearance | White, yellowish white[1], or red[1] crystalline solid |
| Density | 3.95 g/cm3, solid |
| Melting point | 340 ºC (sealed tube) |
| Boiling point | 315 °C subl. |
| 38.4 g/100 mL (20 °C) 39.5 g/100 ml (25 °C) 82.5 g/100 mL (65 °C) | |
| Solubility | soluble in benzene |
| Solubility in ethanol | 6.7 g/100 mL (15 °C) |
| Solubility in acetone | 4.4 g/100 mL (15 °C) |
| Solubility in acetic acid | 1.11 g/100 mL (14 °C) |
| Vapor pressure | 1.65 kPa (70 ºC) |
| Acidity (pKa) | 2.62; 8.32 |
| Structure | |
| see text | |
| trigonal (Se) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Selenium disulfide |
Other cations
|
Sulfur dioxide Tellurium dioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.
Properties
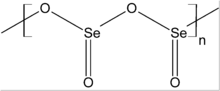
SeO2 is a polar molecule, with the dipole moment pointed from the midpoint of the two oxygen atoms to the selenium atom. Solid SeO2 is a one-dimensional polymer, the chain consisting of alternating selenium and oxygen atoms. Each Se atom, which is pyramidal, bears a terminal oxide group. The relative stereochemistry at Se alternates along the polymer chain (syndiotactic). The solid sublimes readily. The vapour has an odour resembling horseradish sauce and can burn the nose and throat on inhalation. Gaseous selenium dioxide adopts a bent structure very similar to that of sulfur dioxide. Dissolution of SeO2 in selenium oxydichloride give the trimer [Se(O)O]3.[2] Whereas SO2 tends to be molecular and SeO2 is a one-dimensional chain, TeO2 is a cross-linked polymer.
SeO2 is considered an acidic oxide: it dissolves in water to form selenous (selenious) acid. Often the terms selenous acid and selenium dioxide are used interchangeably. It reacts with base to form selenite salts containing the SeO2−
3 anion. For example, reaction with sodium hydroxide produces sodium selenite:
- SeO2 + 2 NaOH → Na2SeO3 + H2O
Preparation
Selenium dioxide is prepared by oxidation of selenium by burning in air, nitric acid or by reaction with hydrogen peroxide, but perhaps the most convenient preparation is by the dehydration of selenous acid.
- 3 Se + 4 HNO3 + H2O → 3 H2SeO3 + 4 NO
- 2 H2O2 + Se → SeO2 + 2 H2O
- H2SeO3 ⇌ SeO2 + H2O
Occurrence
The natural form of selenium dioxide, downeyite, is a very rare mineral. It is found in only a very few burning coal dumps.[3]
Uses
Organic synthesis
SeO2 is an important reagent in organic synthesis. Oxidation of paraldehyde (acetaldehyde trimer) with SeO2 gives glyoxal[4] and the oxidation of cyclohexanone gives cyclohexane-1,2-dione.[5]. The selenium starting material is reduced to selenium, and precipitates as a red amorphous solid which can easily be filtered off.[5] This type of reaction is called a Riley oxidation. It is also renown as a reagent for "allylic" oxidation,[6] a reaction that entails the conversion
- R2C=CR'-CHR"2 + [O] → R2C=CR'-C(OH)R"2
(where R, R', R" are alkyl or aryl).
As a colorant
Selenium dioxide imparts a red colour to glass: it is used in small quantities to counteract the blue colour due to cobalt impurities and so to create (apparently) colourless glass. In larger quantities, it gives a deep ruby red colour.
Selenium dioxide is the active ingredient in some cold-blueing solutions.
It is also used as a toner in photographic developing.
References
- ^ a b http://msds.chem.ox.ac.uk/SE/selenium_dioxide.html
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ^ American Mineralogist. 62: 316–320. 1977 http://www.minsocam.org/ammin/AM62/AM62_316.pdf.
{{cite journal}}: Missing or empty|title=(help); Unknown parameter|unused_data=ignored (help) - ^ Ronzio, A. R.; Waugh, T. D. (1955). "Glyoxal Bisulfite". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 438. - ^ a b Hach, C. C. Banks, C. V.; Diehl, H. (1963). "1,2-Cyclohexanedione Dioxime". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 229. - ^ Coxon, J. M.; Dansted, E.; Hartshorn, M. P. (1988). "Allylic Oxidation with Hydrogen Peroxide–Selenium Dioxide: trans-Pinocarveol". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 946.

