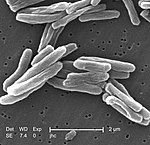
Tuberculosis
 | This article is currently undergoing a major edit by the Guild of Copy Editors. As a courtesy, please do not edit this page while this message is displayed. The copy editor who added this notice is listed in the page history. This page was last revised at 10:46, 17 September 2011 (UTC) (13 years ago) by 59.182.156.56 (talk · contribs) (). Please remove {{GOCEinuse}} from this page as this page has not been edited for at least 24 hours. If you have any questions or concerns, please direct them to the Guild of Copy Editors' talk page. Thank you for your patience. |
This article may require copy editing for Many consecutive sentences starting with "in" or "the", many one- and two-sentence paragraphs. (September 2011) |
and microbiological culture of bodily fluids. Treatment is difficult and requires long courses of multiple antibiotics. Social contacts are also screened and treated if necessary. Antibiotic resistance is a growing problem in (extensively) multi-drug-resistant tuberculosis. Prevention relies on screening programs and vaccination, usually with Bacillus Calmette-Guérin vaccine.
One third of the world's population is thought to be infected with M. tuberculosis,[1][2] and new infections occur at a rate of about one per second.[1] The proportion of people who become sick with tuberculosis each year is stable or falling worldwide but, because of population growth, the absolute number of new cases is still increasing.[1] In 2007 there were an estimated 13.7 million chronic active cases, 9.3 million new cases, and 1.8 million deaths, mostly in developing countries.[3] In addition, more people in the developed world contract tuberculosis because their immune systems are more likely to be compromised due to higher exposure to immunosuppressive drugs, substance abuse, or AIDS. The distribution of tuberculosis is not uniform across the globe; about 80% of the population in many Asian and African countries test positive in tuberculin tests, while only 5–10% of the US population test positive.[4]
Signs and symptoms
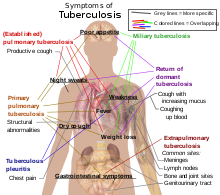
When tuberculosis becomes active, 75% of cases involve infection in the lungs (pulmonary TB). Symptoms include chest pain, coughing up blood, and a productive, prolonged cough for more than three weeks. Systemic symptoms include fever, chills, night sweats, appetite loss, weight loss, pallor, and fatigue.[6]
In the other 25% of active cases, the infection moves from the lungs, causing other kinds of TB, collectively denoted extrapulmonary tuberculosis.[7] This occurs more commonly in immunosuppressed persons and young children. Extrapulmonary infection sites include the pleura in tuberculous pleurisy, the central nervous system in meningitis, the lymphatic system in scrofula of the neck, the genitourinary system in urogenital tuberculosis, and the bones and joints in Pott's disease of the spine. When spread to the bones it is also known as "osseous tuberculosis"[8], a form of Osteomyelitis (as a complication of tuberculosis[4]). An especially serious form is disseminated TB, more commonly known as miliary tuberculosis. Extrapulmonary TB may co-exist with pulmonary TB.[9]
Causes

The main cause of TB, Mycobacterium tuberculosis (MTB), is a small aerobic non-motile bacillus. High lipid content of this pathogen accounts for many of its unique clinical characteristics.[10] It divides every 16 to 20 hours, an extremely slow rate compared with other bacteria, which usually divide in less than an hour.[11] Since MTB has a cell wall but lacks a phospholipid outer membrane, it is classified as a Gram-positive bacterium. However, if a Gram stain is performed, MTB either stains very weakly Gram-positive or does not retain dye as a result of the high lipid and mycolic acid content of its cell wall.[12] MTB can withstand weak disinfectants and survive in a dry state for weeks. In nature, the bacterium can grow only within the cells of a host organism, but M. tuberculosis can be cultured in vitro.[13]
Using histological stains on expectorate samples from phlegm (also called sputum), scientists can identify MTB under a regular microscope. Since MTB retains certain stains after being treated with acidic solution, it is classified as an acid-fast bacillus (AFB).[4][12] The most common acid-fast staining technique, the Ziehl-Neelsen stain, dyes AFBs a bright red that stands out clearly against a blue background. Other ways to visualize AFBs include an auramine-rhodamine stain and fluorescent microscopy.
The M. tuberculosis complex includes four other TB-causing mycobacteria: M. bovis, M. africanum, M. canetti, and M. microti.[14] M. africanum is not widespread, but in parts of Africa it is a significant cause of tuberculosis.[15][16] M. bovis was once a common cause of tuberculosis, but the introduction of pasteurized milk has largely eliminated this as a public health problem in developed countries.[4][17] M. canetti is rare and seems to be limited to Africa, although a few cases have been seen in African emigrants.[18] M. microti is mostly seen in immunodeficient people, although it is possible that the prevalence of this pathogen has been underestimated.[19]
Other known pathogenic mycobacteria include Mycobacterium leprae, Mycobacterium avium, and M. kansasii. The latter two are part of the nontuberculous mycobacteria (NTM) group. Nontuberculous mycobacteria cause neither TB nor leprosy, but they do cause pulmonary diseases resembling TB.[20]
Risk factors
People with silicosis have an approximately 30-fold greater risk for developing TB.[21] Silica particles irritate the respiratory system, causing immunogenic responses such as phagocytosis, which results in high lymphatic vessel deposits.[22] It is probably this interference and blockage of macrophage function that increases the risk of tuberculosis.[23] Persons with chronic renal failure and also on hemodialysis have an increased risk.[24] Persons with diabetes mellitus have a risk for developing active TB that is two to four times greater than persons without diabetes mellitus, and this risk is likely to be greater in persons with insulin-dependent or poorly controlled diabetes.[25] Other clinical conditions that have been associated with active TB include gastrectomy with attendant weight loss and malabsorption, jejunoileal bypass, renal and cardiac transplantation, carcinoma of the head or neck, and other neoplasms (e.g., lung cancer, lymphoma, and leukemia).[21]
Given that silicosis greatly increases the risk of tuberculosis, more research about the effect of various indoor or outdoor air pollutants on the disease would be necessary. Some possible indoor sources of silica include paint, concrete, and Portland cement. Crystalline silica is found in concrete, masonry, sandstone, rock, paint, and other abrasives. The cutting, breaking, crushing, drilling, grinding, or abrasive blasting of these materials may produce fine silica dust. It can also be in soil, mortar, plaster, and shingles.[26]
Low body weight is associated with risk of tuberculosis as well. A body mass index (BMI) below 18.5 increases the risk by 2 to 3 times. An increase in body weight lowers the risk.[27][28] People with diabetes mellitus are at increased risk of contracting tuberculosis,[29] and they have a poorer response to treatment, possibly due to poorer drug absorption.[30]
Diabetes increases the risk of TB three-fold.[31] The correlation between diabetes mellitus and TB is concerning for public health because it shows a distinct connection between a contagious disease and a chronic disease. TB is a highly contagious air-born bacteria. Therefore, contracting tuberculosis depends on whether or not a person comes into contact with the bacteria. Diabetics do not have an increased risk of contracting latent tuberculosis but studies have shown that people with diabetes mellitus are more likely to move from a latent form of TB to an active form of TB. This is where the public concern comes from, because when TB is active it is contagious and potentially fatal.[32]
Other conditions that increase risk include the sharing of needles among IV drug users, recent TB infection or a history of inadequately treated TB, chest X-ray suggestive of previous TB, showing fibrotic lesions and nodules, prolonged corticosteroid therapy and other immunosuppressive therapy, compromised immune system (30–40% of people with AIDS worldwide also have TB), hematologic and reticuloendothelial diseases, such as leukemia and Hodgkin's disease, end-stage kidney disease, intestinal bypass, chronic malabsorption syndromes, vitamin D deficiency,[33] and low body weight.[4][9]
Twin studies in the 1940s showed that susceptibility to TB was heritable. If one of a pair of twins got TB, then the other was more likely to get TB if he was identical than if he was not.[34] These findings were more recently confirmed by a series of studies in South Africa.[35][36][37] Specific gene polymorphisms in IL12B have been linked to tuberculosis susceptibility.[38]
Some drugs, including rheumatoid arthritis drugs that work by blocking tumor necrosis factor-alpha (an inflammation-causing cytokine), raise the risk of activating a latent infection due to the importance of this cytokine in the immune defense against TB.[39]
Mechanism
Transmission
When people suffering from active pulmonary TB cough, sneeze, speak, sing, or spit, they expel infectious aerosol droplets 0.5 to 5 µm in diameter. A single sneeze can release up to 40,000 droplets.[40] Each one of these droplets may transmit the disease, since the infectious dose of tuberculosis is very low and inhaling fewer than ten bacteria may cause an infection.[41][42]
People with prolonged, frequent, or intense contact are at particularly high risk of becoming infected, with an estimated 22% infection rate. A person with active but untreated tuberculosis can infect 10–15 other people per year.[1] Others at risk include people in areas where TB is common, people who inject illicit drugs, residents and employees of high-risk congregate settings, medically under-served and low-income populations, high-risk racial or ethnic minority populations, children exposed to adults in high-risk categories, those who are immunocompromised by conditions such as HIV/AIDS, people who take immunosuppressant drugs, and health care workers serving these high-risk clients.[43]
Transmission can only occur from people with active—not latent—TB.[4] The probability of transmission from one person to another depends upon the number of infectious droplets expelled by a carrier, the effectiveness of ventilation, the duration of exposure, and the virulence of the M. tuberculosis strain.[9] The chain of transmission can be broken by isolating people with active disease and starting effective anti-tuberculous therapy. After two weeks of such treatment, people with non-resistant active TB generally cease to be contagious. If someone does become infected, then it will take three to four weeks before the newly infected person can transmit the disease to others.[44]
Pathogenesis
About 90% of those infected with Mycobacterium tuberculosis have asymptomatic, latent TB infection (sometimes called LTBI), with only a 10% lifetime chance that a latent infection will progress to TB disease.[4] However, if untreated, the death rate for these active TB cases is more than 50%.[1]
TB infection begins when the mycobacteria reach the pulmonary alveoli, where they invade and replicate within the endosomes of alveolar macrophages.[4][45] The primary site of infection in the lungs is called the Ghon focus, and is generally located in either the upper part of the lower lobe, or the lower part of the upper lobe.[4] Bacteria are picked up by dendritic cells, which do not allow replication, although these cells can transport the bacilli to local (mediastinal) lymph nodes. Further spread is through the bloodstream to other tissues and organs where secondary TB lesions can develop in other parts of the lung (particularly the apex of the upper lobes), peripheral lymph nodes, kidneys, brain, and bone.[4][46] All parts of the body can be affected by the disease, though it rarely affects the heart, skeletal muscles, pancreas and thyroid.[47]
Tuberculosis is classified as one of the granulomatous inflammatory conditions. Macrophages, T lymphocytes, B lymphocytes, and fibroblasts are among the cells that aggregate to form granulomas, with lymphocytes surrounding the infected macrophages. The granuloma prevents dissemination of the mycobacteria and provides a local environment for interaction of cells of the immune system. Bacteria inside the granuloma can become dormant, resulting in a latent infection. Another feature of the granulomas of human tuberculosis is the development of abnormal cell death (necrosis) in the center of tubercles. To the naked eye this has the texture of soft white cheese and is termed caseous necrosis.[48]
If TB bacteria gain entry to the bloodstream from an area of damaged tissue they spread through the body and set up many foci of infection, all appearing as tiny white tubercles in the tissues. This severe form of TB disease is most common in infants and the elderly and is called miliary tuberculosis. People with this disseminated TB have a fatality rate near 100% if untreated. However, If treated early, the fatality rate is reduced to about 10%.[49]
In many people the infection waxes and wanes. Tissue destruction and necrosis are balanced by healing and fibrosis.[48] Affected tissue is replaced by scarring and cavities filled with cheese-like white necrotic material. During active disease, some of these cavities are joined to the air passages bronchi and this material can be coughed up. It contains living bacteria and can therefore pass on infection. Treatment with appropriate antibiotics kills bacteria and allows healing to take place. Upon cure, affected areas are eventually replaced by scar tissue.[48]
If untreated, infection with Mycobacterium tuberculosis can cause lobar pneumonia.[50]
Diagnosis
Tuberculosis is diagnosed definitively by identifying the causative organism (Mycobacterium tuberculosis) in a clinical sample (for example, sputum or pus). When this is not possible, a probable—although sometimes inconclusive[51]—diagnosis may be made using imaging (X-rays or scans), a tuberculin skin test (Mantoux test),[51] or a, Interferon Gamma Release Assay (IGRA).
The main problem with tuberculosis diagnosis is the difficulty in culturing this slow-growing organism in the laboratory (it may take 4 to 12 weeks for blood or sputum culture). A complete medical evaluation for TB must include a medical history, a physical examination, a chest X-ray, microbiological smears, and cultures. It may also include a tuberculin skin test, a serological test. The interpretation of the tuberculin skin test depends upon the person's risk factors for infection and progression to TB disease, such as exposure to other cases of TB or immunosuppression.[9]
Currently, latent infection is diagnosed in a non-immunized person by a tuberculin skin test, which yields a delayed hypersensitivity type response to an extract made from M. tuberculosis.[4] Those immunized for TB or with past-cleared infection will respond with delayed hypersensitivity parallel to those currently in a state of infection, so the test must be used with caution, particularly with regard to persons from countries where TB immunization is common.[52] Tuberculin tests have the disadvantage of producing false negatives, especially when the person is co-morbid with sarcoidosis, Hodgkins lymphoma, malnutrition, or most notably active tuberculosis disease.[4] The newer interferon release assays (IGRAs) such as T-SPOT.TB and QuantiFERON-TB Gold In Tube overcome many of these problems. IGRAs are in vitro blood tests that are more specific than the skin test. IGRAs detect the release of interferon gamma in response to mycobacterial proteins such as ESAT-6.[53] These are not affected by immunization or environmental mycobacteria, so generate fewer false positive results.[54] There is also evidence that IGRAs are more sensitive than the skin test.[55]
New TB tests have been developed that are fast and accurate. These include polymerase chain reaction assays for the detection of bacterial DNA.[56] One such molecular diagnostics test gives results in 100 minutes and is currently being offered to 116 low- and middle-income countries at a discount with support from WHO and the Bill and Melinda Gates foundation.[57]
Another such test, which was approved by the FDA in 1996, is the amplified mycobacterium tuberculosis direct test (MTD, Gen-Probe). This test yields results in 2.5 to 3.5 hours, and it is highly sensitive and specific when used to test smears positive for acid-fast bacilli (AFB).[58]
Screening

Mantoux tuberculin skin tests are often used for routine screening of high risk individuals.[59] Interferon-γ release assays are blood tests used in the diagnosis of some infectious diseases. There are currently two interferon-γ release assays available for the diagnosis of tuberculosis:
- QuantiFERON-TB Gold (licensed in US, Europe, and Japan); and
- T-SPOT.TB, a form of ELISPOT (licensed in Europe).
Chest photofluorography has been used in the past for mass screening for tuberculosis.
Prevention

TB prevention and control takes two parallel approaches. In the first, people with TB and their contacts are identified and then treated. Identification of infections often involves testing high-risk groups for TB. In the second approach, children are vaccinated to protect them from TB. No vaccine is available that provides reliable protection for adults. However, in tropical areas where the levels of other species of mycobacteria are high, exposure to nontuberculous mycobacteria gives some protection against TB.[60]
The World Health Organization (WHO) declared TB a global health emergency in 1993, and the Stop TB Partnership developed a Global Plan to Stop Tuberculosis that aims to save 14 million lives between 2006 and 2015.[61] Since humans are the only host of Mycobacterium tuberculosis, eradication would be possible. This goal would be helped greatly by an effective vaccine.[62]
Vaccines
Many countries use the Bacillus Calmette-Guérin (BCG) vaccine as part of their TB control programmes, especially for infants. The BCG vaccine is one of the most widely used of all current vaccines, reaching >80% of neonates and infants in countries with a national vaccination schedule.[63] In the US, where TB is uncommon, BCG is not widely administered.[64] BCG was the first vaccine for TB. From 1905, Albert Calmette and Camille Guérin worked at the Institut Pasteur de Lille and the Pasteur Institute in France developing BCG, administering the first human trials in 1921.[65] However, deaths due to flawed manufacturing processes created public resistance to BCG, delaying mass vaccinations until after World War II.[66] The protective efficacy of BCG for preventing serious forms of TB (e.g. meningitis) in children is greater than 80%. Its protective efficacy for preventing pulmonary TB in adolescents and adults varies by country (as low as 0% in South India); in the United Kingdom, its effectiveness exceeds 75%.[67]
In South Africa, the country with the highest prevalence of TB, BCG is given to all children under age three.[68] However, BCG is less effective in areas where mycobacteria are less prevalent; therefore BCG is not given to the entire population in such countries. In the USA, for example, BCG vaccine is not recommended except for people who meet specific criteria:[9]
- Infants or children with negative skin test results who are continually exposed to untreated or ineffectively treated people or will be continually exposed to multi-drug-resistant tuberculosis (MDR-TB).
- Healthcare workers considered on an individual basis in settings in which a high percentage of MDR-TB has been found, transmission of MDR-TB is likely, and TB control precautions have been implemented and were not successful.
BCG provides some protection against severe forms of pediatric TB, but has been shown to be unreliable against adult pulmonary TB, which accounts for most of the disease burden worldwide. Currently, there are more cases of TB on the planet than at any other time in history and most agree there is an urgent need for a newer, more effective vaccine that would prevent all forms of TB—including drug resistant strains—in all age groups and among people with HIV.[69]
Several new vaccines to prevent TB infection are being developed, among others by Aeras and TBVI. The first recombinant tuberculosis vaccine, Mtb72F, entered clinical trials in the United States in 2004, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID).[70][71] A 2005 study showed that a DNA TB vaccine given with conventional chemotherapy can accelerate the disappearance of bacteria as well as protect against re-infection in mice; it may take four to five years to be available in humans.[72] Another TB vaccine, MVA85A, is currently in phase II trials in South Africa,[73] and is based on a genetically modified vaccinia virus. Many other strategies are also being used to develop novel vaccines,[74] including both subunit vaccines (fusion molecules composed of two recombinant proteins delivered in an adjuvant) such as Hybrid-1, HyVac4, or M72, and recombinant adenoviruses such as Ad35.[75][76][77][78] Some of these vaccines can be effectively administered without needles, making them preferable for areas where HIV is common.[79] All of these vaccines have been successfully tested in humans and are now in extended testing in TB-endemic regions. To encourage further discovery, researchers and policymakers are promoting new economic models of vaccine development including prizes, tax incentives, and advance market commitments.[80][81]
An experimental vaccine, with positive results in mouse models, may be effective in not only preventing infection, but also in eradicating the infection once established.[82] A tuberculosis vaccine aimed at sterile Mtb eradication should be able to target latent Mtb as well as Mtb that causes early-stage tuberculosis.[83] The vaccine is a combination of antigens Ag85B and ESAT-6 as well as the protein Rv2660c. Ag85B and ESAT-6 together form the vaccine Hybrid-1, while Rv2660c is a protein that is expressed even in late-stage infections, when protein transcription is generally reduced. The novel combination of Ag85B, ESAT-6, and Rv2660c allows for both short- and long-term protection as a result of the continued expression of target proteins. The new vaccine, currently referred to as H56, works by promoting a polyfunctional CD4+ T cell response against tuberculosis protein components. Phase I clinical trials are scheduled to begin in Cape Town, South Africa, in March 2011.[82][needs update]
Treatment
Treatment for TB uses antibiotics to kill the bacteria. Effective TB treatment is difficult, due to the unusual structure and chemical composition of the mycobacterial cell wall, which makes many antibiotics ineffective and hinders the entry of drugs.[84][85][86][87] The two antibiotics most commonly used are isoniazid and rifampicin. However, instead of the short course of antibiotics typically used to cure other bacterial infections, TB requires much longer periods of treatment (around 6 to 24 months) to entirely eliminate mycobacteria from the body.[9] Latent TB treatment usually uses a single antibiotic, while active TB disease is best treated with combinations of several antibiotics, to reduce the risk of the bacteria developing antibiotic resistance.[88] People with latent infections are treated to prevent them from progressing to active TB disease later in life.
Drug-resistant tuberculosis is transmitted in the same way as regular TB. Primary resistance occurs in persons infected with a resistant strain of TB. A person with fully susceptible TB develops secondary resistance (acquired resistance) during TB therapy because of inadequate treatment, not taking the prescribed regimen appropriately, or using low-quality medication.[88] Drug-resistant TB is a public health issue in many developing countries, as treatment is longer and requires more expensive drugs. Multi-drug-resistant tuberculosis (MDR-TB) is defined as resistance to the two most effective first-line TB drugs: rifampicin and isoniazid. Extensively drug-resistant TB (XDR-TB) is also resistant to three or more of the six classes of second-line drugs.[89]
The DOTS (Directly Observed Treatment Short-course) strategy of tuberculosis treatment recommended by WHO was based on clinical trials done in the 1970s by the Tuberculosis Research Centre in Chennai, India. The country in which a person with TB lives can determine what treatment they receive. This is because multi-drug-resistant tuberculosis is resistant to most first-line medications, so the use of second-line antituberculosis medications is necessary to cure the person. However, the price of these medications is high; thus, poor people in the developing world have no or limited access to these treatments.[90]
In the early 1900s to 1950s doctors would try to collapse the infected lung by breaking several ribs or inflating that half of the chest with air.[91]
Prognosis
Progression from TB infection to TB disease occurs when the TB bacilli overcome the immune system defenses and begin to multiply. In primary TB disease—1–5% of cases—this occurs soon after infection.[4] However, in the majority of cases, a latent infection occurs that has no obvious symptoms.[4] These dormant bacilli can produce tuberculosis in 2–23% of these latent cases, often many years after infection.[92]
The risk of reactivation increases with immunosuppression, such as that caused by infection with HIV. In people co-infected with M. tuberculosis and HIV, the risk of reactivation increases to 10% per year.[4] Studies utilizing DNA fingerprinting of M. tuberculosis strains have shown that reinfection contributes more substantially to recurrent TB than previously thought,[93] with between 12% and 77% of cases attributable to reinfection (instead of reactivation).[94]
Epidemiology



Roughly a third of the world's population has been infected with M. tuberculosis, and new infections occur at a rate of one per second.[1] However, not all infections with M. tuberculosis cause TB disease and many infections are asymptomatic.[98] In 2007, an estimated 13.7 million people had active TB disease, with 9.3 million new cases and 1.8 million deaths; the annual incidence rate varied from 363 per 100,000 in Africa to 32 per 100,000 in the Americas.[3] Tuberculosis is the world's greatest infectious killer of women of reproductive age and the leading cause of death among people with HIV/AIDS.[99]
The rise in HIV infections and the neglect of TB control programs have enabled a resurgence of tuberculosis.[100] The emergence of drug-resistant strains has also contributed to this new epidemic with, from 2000 to 2004, 20% of TB cases being resistant to standard treatments and 2% resistant to second-line drugs.[89] The rate at which new TB cases occur varies widely, even in neighbouring countries, apparently because of differences in health care systems.[101]
In 2007, the country with the highest estimated incidence rate of TB was Swaziland, with 1200 cases per 100,000 people. India had the largest total incidence, with an estimated 2.0 million new cases.[3] In developed countries, tuberculosis is less common and is mainly an urban disease. In the United Kingdom, the national average was 15 per 100,000 in 2007, and the highest incidence rates in Western Europe were 30 per 100,000 in Portugal and Spain. These rates compared with 98 per 100,000 in China and 48 per 100,000 in Brazil. In the United States, the overall tuberculosis case rate was 4 per 100,000 persons in 2007.[96] In Canada, tuberculosis is still endemic in some rural areas.[102]
The incidence of TB varies with age. In Africa, TB primarily affects adolescents and young adults.[103] However, in countries where TB has gone from high to low incidence, such as the United States, TB is mainly a disease of older people, or of the immunocompromised.[4][104]
There are a number of known factors that make people more susceptible to TB infection; worldwide the most important of these is HIV. Co-infection with HIV is a particular problem in Sub-Saharan Africa, due to the high incidence of HIV in these countries.[105][106] Smoking more than 20 cigarettes a day increases the risk of TB by two to four times.[107][108] Diabetes mellitus is also an important risk factor that is growing in importance in developing countries.[109] Other disease states that increase the risk of developing tuberculosis are Hodgkin lymphoma, end-stage renal disease, chronic lung disease, malnutrition, and alcoholism.[4]
Diet may also modulate risk. For example, among immigrants in London from the Indian subcontinent, vegetarian Hindu Asians were found to have an 8.5 fold increased risk of tuberculosis, compared to Muslims who ate meat and fish daily.[110] Although a causal link is not proved by this data,[111] this increased risk could be caused by micronutrient deficiencies: possibly iron, vitamin B12 or vitamin D.[110] Further studies have provided more evidence of a link between vitamin D deficiency and an increased risk of contracting tuberculosis.[112][113] Globally, the severe malnutrition common in parts of the developing world causes a large increase in the risk of developing active tuberculosis, due to its damaging effects on the immune system.[114][115] Along with overcrowding, poor nutrition may contribute to the strong link observed between tuberculosis and poverty.[116][117]
Prisoners, especially in poor countries, are particularly vulnerable to infectious diseases such as HIV/AIDS and TB. Prisons provide conditions that allow TB to spread rapidly due to overcrowding, poor nutrition, and a lack of health services. Since the early 1990s, TB outbreaks have been reported in prisons in many countries in Eastern Europe. The prevalence of TB in prisons is much higher than among the general population—in some countries as much as 40 times higher.[118][119]
History

Tuberculosis has been present in humans since antiquity. The earliest unambiguous detection of Mycobacterium tuberculosis is in the remains of bison dated 18,000 years before the present.[120] Whether tuberculosis originated in cattle and then transferred to humans, or diverged from a common ancestor infecting a different species, is currently unclear.[121] However, it is clear that M. tuberculosis is not directly descended from M. bovis, which seems to have evolved relatively recently.[122]
Skeletal remains from a Neolithic Settlement in the Eastern Mediterranean show prehistoric humans (7000 BC) had TB,[123] and tubercular decay has been found in the spines of mummies from 3000–2400 BC.[124] Phthisis is a Greek term for tuberculosis; around 460 BC, Hippocrates identified phthisis as the most widespread disease of the times involving coughing up blood and fever, which was almost always fatal.[125] In South America, the earliest evidence of tuberculosis is associated with the Paracas-Caverna culture (circa 750 BC to circa 100 AD).[126][127] Jane E. Buikstra deduced from skeletal remains that "virtually every member" of these late prehistoric North American communities were exposed to tuberculosis.[128]
Other names
In the past, tuberculosis has been called consumption, because it seemed to consume people from within, with a bloody cough, fever, pallor, and long relentless wasting. Other names included phthisis (Greek for consumption) and phthisis pulmonalis; scrofula (in adults), affecting the lymphatic system and resulting in swollen neck glands; tabes mesenterica, TB of the abdomen and lupus vulgaris, TB of the skin; wasting disease; white plague, because sufferers appear markedly pale; king's evil, because it was believed that a king's touch would heal scrofula; and Pott's disease, or gibbus of the spine and joints.[129][130]

Miliary tuberculosis—now commonly known as disseminated TB—occurs when the infection invades the circulatory system, resulting in millet-like seeding of TB bacilli in the lungs as seen on an X-ray.[129][131] TB is also called Koch's disease, after the scientist Robert Koch.[132]
Folklore
Before the Industrial Revolution, tuberculosis was sometimes regarded as vampirism. When one member of a family died from it, the other members that were infected would lose their health slowly. Folklore held that this was caused by the original victim draining the life from the other family members. Furthermore, people who had TB exhibited symptoms similar to what people considered to be vampire traits. People with TB often have symptoms such as red, swollen eyes (which also creates a sensitivity to bright light), pale skin, extremely low body heat, a weak heart and coughing blood, suggesting the idea that the only way for the afflicted to replenish this loss of blood was by sucking blood.[133] Another folk belief told that the affected individual was being forced, nightly, to attend fairy revels, so that the victim wasted away owing to lack of rest; this belief was most common when a strong connection was seen between the fairies and the dead.[134] Similarly, but less commonly, it was attributed to the victims being "hagridden"—being transformed into horses by witches (hags) to travel to their nightly meetings, again resulting in a lack of rest.[134]
TB was romanticized in the nineteenth century. Many people believed TB produced feelings of euphoria referred to as Spes phthisica ("hope of the consumptive"). It was believed that TB sufferers who were artists had bursts of creativity as the disease progressed. It was also believed that TB sufferers acquired a final burst of energy just before they died that made women more beautiful and men more creative.[135][136]
Study and treatment
In ancient times, treatments focused on sufferers' diets. Pliny the Elder described several methods in his Natural History: "wolf's liver taken in thin wine, the lard of a sow that has been fed upon grass, or the flesh of a she-ass taken in broth".[137] Ibn Sina (Avicenna) wrote on tuberculosis in the 1020s in his The Canon of Medicine. He adopted, from the Greeks, the theory that epidemics are caused by pollution in the air (miasma, a noxious form of "bad air").[138]
Although it was established that the pulmonary form was associated with "tubercles" by Dr Richard Morton in 1689,[139][140] due to the variety of its symptoms, TB was not identified as a single disease until the 1820s and was not named "tuberculosis" until 1839 by J. L. Schönlein.[141] Between 1838 and 1845, Dr. John Croghan, the owner of Mammoth Cave, brought a number of tuberculosis sufferers into the cave in the hope of curing the disease with the constant temperature and purity of the cave air; they died within a year.[142] The first TB sanatorium opened in 1854 in Görbersdorf, Germany (today Sokołowsko, Poland) by Hermann Brehmer.[143]
The bacillus causing tuberculosis, Mycobacterium tuberculosis, was identified and described on 24 March 1882 by Robert Koch. He received the Nobel Prize in physiology or medicine in 1905 for this discovery.[144] Koch did not believe that bovine (cattle) and human tuberculosis were similar, which delayed the recognition of infected milk as a source of infection. Later, this source was eliminated by the pasteurization process. Koch announced a glycerine extract of the tubercle bacilli as a remedy for tuberculosis in 1890, calling it "tuberculin". It was not effective, but was later adapted as a test for pre-symptomatic tuberculosis.[145]
The first genuine success in immunizing against tuberculosis was developed from attenuated bovine-strain tuberculosis by Albert Calmette and Camille Guérin in 1906. It was called "BCG" (Bacillus of Calmette and Guérin). The BCG vaccine was first used on humans in 1921 in France,[65] but it was not until after World War II that BCG received widespread acceptance in the USA, Great Britain, and Germany.[66]
Tuberculosis, or "consumption" as it was commonly known, caused the most widespread public concern in the 19th and early 20th centuries as an endemic disease of the urban poor.[146] In 1815, one in four deaths in England was of consumption; by 1918 one in six deaths in France were still caused by TB. In the 20th century, tuberculosis killed an estimated 100 million people.[147] After the establishment in the 1880s that the disease was contagious, TB was made a notifiable disease in Britain; there were campaigns to stop spitting in public places, and the infected poor were pressured to enter sanatoria that resembled prisons; the sanatoria for the middle and upper classes offered excellent care and constant medical attention.[143] Whatever the purported benefits of the fresh air and labor in the sanatoria, even under the best conditions, 50% of those who entered were dead within five years (1916).[143]

The promotion of Christmas Seals began in Denmark during 1904 as a way to raise money for tuberculosis programs. It expanded to the United States and Canada in 1907–1908 to help the National Tuberculosis Association (later called the American Lung Association).
In the United States, concern about the spread of tuberculosis played a role in the movement to prohibit public spitting except into spittoons.
In Europe, deaths from TB fell from 500 out of 100,000 in 1850 to 50 out of 100,000 by 1950. Improvements in public health were reducing tuberculosis even before the arrival of antibiotics. The disease remained such a significant threat to public health, that when the Medical Research Council was formed in Britain in 1913, its initial focus was tuberculosis research.[148]
It was not until 1946, with the development of the antibiotic streptomycin, that effective treatment and cure became possible. Prior to the introduction of this drug, the only treatments besides sanatoria were surgical interventions, including bronchoscopy and suction as well as the pneumothorax or plombage technique—collapsing an infected lung to "rest" it and allow lesions to heal—a technique that was of little benefit and was mostly discontinued by the 1950s.[149] The emergence of multi-drug-resistant TB has again introduced surgery as part of the treatment for these infections. Here, surgical removal of infected nodules will reduce the number of bacteria in the lungs, as well as increasing the exposure of the remaining bacteria to drugs in the bloodstream. It is therefore thought to increase the effectiveness of the chemotherapy.[150]
Hopes that the disease could be completely eliminated have been dashed since the rise of drug-resistant strains in the 1980s. For example, tuberculosis cases in Britain, numbering around 117,000 in 1913, had fallen to around 5,000 in 1987, but cases rose again, reaching 6,300 in 2000 and 7,600 cases in 2005.[151] Due to the elimination of public health facilities in New York and the emergence of HIV, there was a resurgence of TB in the late 1980s.[152] The number of people failing to complete their course of drugs is high. New York had to cope with more than 20,000 people with multidrug-resistant strains of TB (resistant to, at least, both Rifampin and Isoniazid).
The resurgence of tuberculosis resulted in the declaration of a global health emergency by the World Health Organization (WHO) in 1993.[153] Every year, nearly half a million new cases of multi-drug-resistant tuberculosis (MDR-TB) are estimated to occur worldwide.[154]
Age
The oldest known human remains showing signs of tuberculosis infection are over 9,000 years old.[123] During this period, M. tuberculosis has lost numerous coding and non-coding regions in its genome, losses that can be used to distinguish between strains of the bacteria. The implication is that M. tuberculosis strains differ geographically, so their genetic differences can be used to track the origins and movement of each strain.[155]
Society and culture
Since it has affected important historical figures, tuberculosis has influenced particularly European history, and become a theme in art—mostly literature, music, and film. Most of this was due to others seeing a final expression of beauty and creativity coming from those who were infected.[156]
Public health
Tuberculosis is one of the three primary diseases of poverty along with AIDS and malaria.[157] The Global Fund to Fight AIDS, Tuberculosis and Malaria was started in 2002 to raise finances to address these infectious diseases. Globalization has led to increased opportunities for disease spread. A tuberculosis scare occurred in 2007 when Andrew Speaker flew on a transatlantic flight infected with multi-drug-resistant tuberculosis.[158]
Globally, the World Health Organization and its Stop TB strategy are leading public health efforts to reduce the global burden of tuberculosis by 2015.[159] In the United States, the National Center for HIV, STD, and TB Prevention, as part of the Centers for Disease Control and Prevention (CDC), is responsible for public health surveillance and prevention research.[160]
In 2011, Public Health Law Research published an evidence brief summarizing the research assessing the effect of a specific law or policy on public health, The Effects of Laws Authorizing Coercive Tuberculosis Control Measures. They stated that "there is insufficient evidence at this time to conclude that detention of individuals with infectious tuberculosis improves population health."[161]
In other animals
Tuberculosis can be carried by mammals; domesticated species, such as cats and dogs, are generally free of tuberculosis, but wild animals may be carriers.
Mycobacterium bovis causes TB in cattle. An effort to eradicate bovine tuberculosis from the cattle and deer herds of New Zealand is under way. It has been found that herd infection is more likely in areas where infected natural reservoirs such as Australian brush-tailed possums come into contact with domestic livestock at farm-bush borders.[162] Controlling the vectors through possum eradication and monitoring the level of disease in livestock herds through regular surveillance form a "two-pronged" approach to ridding New Zealand of the disease.
In Ireland and the United Kingdom, badgers have been identified as one vector species for the transmission of bovine tuberculosis. As a result, governments have come under pressure from some quarters, primarily dairy farmers, to mount an active campaign of eradication of badgers in certain areas with the purpose of reducing the incidence of bovine TB. The effectiveness of culling on the incidence of TB in cattle is a contentious issue, with proponents and opponents citing their own studies to support their position.[163] For instance, a study by an Independent Study Group on badger culling reported on 18 June 2007 that it was unlikely to be effective and would only make a “modest difference” to the spread of TB, and that "badger culling cannot meaningfully contribute to the future control of cattle TB"; in contrast, another report concluded that this policy would have a significant impact.[164] On 4 July 2008, the UK government decided against a proposed random culling policy.[165]
See also
References
- ^ a b c d e f "Tuberculosis Fact sheet N°104". World Health Organization. November 2010. Retrieved 26 July 2011.
- ^ Jasmer RM, Nahid P, Hopewell PC (2002). "Clinical practice. Latent tuberculosis infection" (PDF). N. Engl. J. Med. 347 (23): 1860–6. doi:10.1056/NEJMcp021045. PMID 12466511.
{{cite journal}}: CS1 maint: multiple names: authors list (link), which cites Dolin PJ, Raviglione MC, Kochi A (1994). "Global tuberculosis incidence and mortality during 1990–2000". Bull World Health Organ. 72 (2): 213–20. PMC 2486541. PMID 8205640.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c World Health Organization (2009). "Epidemiology". Global tuberculosis control: epidemiology, strategy, financing. pp. 6–33. ISBN 9789241563802.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ a b c d e f g h i j k l m n o p q Cite error: The named reference
Robbinswas invoked but never defined (see the help page). - ^ Schiffman G (15 January 2009). "Tuberculosis Symptoms". eMedicineHealth.
- ^ "Tuberculosis (TB) Symptoms". NHS Choices Tuberculosis. National Health Service (NHS) UK. Retrieved 17 April 2011.
- ^ Golden MP, Vikram HR (2005). "Extrapulmonary tuberculosis: an overview". American family physician. 72 (9): 1761–8. PMID 16300038.
- ^ Mile WA (1974 Sep). "Osseous tuberculosis". J Natl Med Assoc. 66 (5): 400–3. PMID 4414957.
{{cite journal}}: Check date values in:|year=(help)CS1 maint: year (link) - ^ a b c d e f "Core Curriculum on Tuberculosis: What the Clinician Should Know" (4th edition ed.). Centers for Disease Control and Prevention (CDC), Division of Tuberculosis Elimination. 2000, updated August 2003.
{{cite web}}:|edition=has extra text (help); Check date values in:|year=(help)CS1 maint: year (link) - ^ Southwick F (10 December 2007). "Chapter 4: Pulmonary Infections". Infectious Diseases: A Clinical Short Course, 2nd ed. McGraw-Hill Medical Publishing Division. p. 104. ISBN 0071477225.
{{cite book}}: More than one of|pages=and|page=specified (help) - ^ Cox R (2004). "Quantitative relationships for specific growth rates and macromolecular compositions of Mycobacterium tuberculosis, Streptomyces coelicolor A3(2) and Escherichia coli B/r: an integrative theoretical approach". Microbiology. 150 (Pt 5): 1413–26. doi:10.1099/mic.0.26560-0. PMID 15133103.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Madison B (2001). "Application of stains in clinical microbiology". Biotech Histochem. 76 (3): 119–25. doi:10.1080/714028138. PMID 11475314.
- ^ Parish T, Stoker N (1999). "Mycobacteria: bugs and bugbears (two steps forward and one step back)". Mol Biotechnol. 13 (3): 191–200. doi:10.1385/MB:13:3:191. PMID 10934532.
- ^ van Soolingen D; et al. (1997). "A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa". Int. J. Syst. Bacteriol. 47 (4): 1236–45. doi:10.1099/00207713-47-4-1236. PMID 9336935.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Niemann S; et al. (2002). "Mycobacterium africanum subtype II is associated with two distinct genotypes and is a major cause of human tuberculosis in Kampala, Uganda". J. Clin. Microbiol. 40 (9): 3398–405. doi:10.1128/JCM.40.9.3398-3405.2002. PMC 130701. PMID 12202584.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Niobe-Eyangoh SN; et al. (2003). "Genetic biodiversity of Mycobacterium tuberculosis complex strains from persons with pulmonary tuberculosis in Cameroon". J. Clin. Microbiol. 41 (6): 2547–53. doi:10.1128/JCM.41.6.2547-2553.2003. PMC 156567. PMID 12791879.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Thoen C, Lobue P, de Kantor I (2006). "The importance of Mycobacterium bovis as a zoonosis". Vet. Microbiol. 112 (2–4): 339–45. doi:10.1016/j.vetmic.2005.11.047. PMID 16387455.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pfyffer GE, Auckenthaler R, van Embden JD, van Soolingen D (1998). "Mycobacterium canettii, the smooth variant of M. tuberculosis, isolated from a Swiss patient exposed in Africa". Emerging Infect. Dis. 4 (4): 631–4. doi:10.3201/eid0404.980414. PMC 2640258. PMID 9866740.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Niemann S, Richter E, Dalügge-Tamm H, Schlesinger H, Graupner D, Königstein B, Gurath G, Greinert U, Rüsch-Gerdes S (2000). "Two cases of Mycobacterium microti derived tuberculosis in HIV-negative immunocompetent patients". Emerg Infect Dis. 6 (5): 539–42. doi:10.3201/eid0605.000516. PMC 2627952. PMID 10998387.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association". Am J Respir Crit Care Med. 156 (2 Pt 2): S1–25. 1997. PMID 9279284.
- ^ a b "Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection". cdc.gov. Retrieved 13 April 2010.
- ^ Lee JH (1948). "Tuberculosis And Silicosis". Can Med Assoc J. 58 (4): 349–353. PMC 1591092. PMID 18916106.
- ^ Varkey B (26 January 2011). "Silicosis". WebMD. Retrieved 26 July 2011.
- ^ Segall L, Covic A (2010). "Diagnosis of tuberculosis in dialysis patients: current strategy". Clinical Journal of the American Society of Nephrology : CJASN. 5 (6): 1114–22. doi:10.2215/CJN.09231209. PMID 20413440.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Jeon CY, Murray MB (2008). Williams, Brian (ed.). "Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies". PLoS Medicine. 5 (7): e152. doi:10.1371/journal.pmed.0050152. PMC 2459204. PMID 18630984.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ "NIOSH – Silicosis: Learn the Facts!". cdc.gov. Retrieved 13 April 2010.
- ^ "Arch Intern Med – Leung et al. 167 (12): 1297 Figure OI70054T5". archinte.ama-assn.org. Retrieved 13 April 2010.
- ^ "Arch Intern Med – Leung et al. 167 (12): 1297 Figure OI70054F1". archinte.ama-assn.org. Retrieved 13 April 2010.
- ^ Restrepo, BI (2007). "Convergence of the tuberculosis and diabetes epidemics: Renewal of old acquaintances". Clin Infect Dis. 45 (4): 436–438. doi:10.1086/519939. PMC 2900315. PMID 17638190.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ Nijland HMJ; et al. (2006). "Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes". Clin Infect Dis. 43 (7): 848–854. doi:10.1086/507543. PMID 16941365.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Stevenson CR, Forouhi NG, Roglic G; et al. (2007). "Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence". BMC Public Health. 7: 234. doi:10.1186/1471-2458-7-234. PMID 17822539.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Dooley KE, Chaisson RE (2009). "Tuberculosis and diabetes mellitus: convergence of two epidemics". Lancet Infect Dis. 9 (12): 737–46. doi:10.1016/S1473-3099(09)70282-8. PMC 2945809. PMID 19926034.
- ^ Nnoaham KE, Clarke A (2008). "Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis". Int J Epidemiol. 37 (1): 113–19. doi:10.1093/ije/dym247. PMID 18245055.
- ^ Kallmann FJ, Reisner D (1942). "Twin studies on the significance of genetic factors in tuberculosis". Am Rev Tuberc. 16: 593–617.
- ^ Jepson A; et al. (2001). "Genetic regulation of acquired immune responses to antigens of Mycobacterium tuberculosis: a study of twins in West Africa". Infect Immun. 69 (6): 3989–94. doi:10.1128/IAI.69.6.3989-3994.2001. PMC 98461. PMID 11349068.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Sepulveda RL, Heiba IM, Navarrete C, Elston RC, Gonzalez B, Sorensen RU (1994). "Tuberculin reactivity after newborn BCG immunization in mono‐ and dizygotic twins". Tuber Lung Dis. 75 (2): 138–43. doi:10.1016/0962-8479(94)90043-4. PMID 8032047.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cobat A; et al. (2010). "High heritability of antimycobacterial immunity in an area of hyperendemicity for tuberculosis disease". J Infect Dis. 201 (1): 15–19. doi:10.1086/648611. PMID 19938975.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Tso HW, Lau YL, Tam CM, Wong HS, Chiang KS (2004). "Associations between IL12B polymorphisms and tuberculosis in the Hong Kong Chinese population". J Infect Dis. 190 (5): 913–9. doi:10.1086/422693. PMID 15295696.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mutlu G, Mutlu E, Bellmeyer A, Rubinstein I (2006). "Pulmonary adverse events of anti-tumor necrosis factor-alpha antibody therapy". Am J Med. 119 (8): 639–46. doi:10.1016/j.amjmed.2006.01.015. PMID 16887405.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cole E, Cook C (1998). "Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies". Am J Infect Control. 26 (4): 453–64. doi:10.1016/S0196-6553(98)70046-X. PMID 9721404.
- ^ Nicas M, Nazaroff WW, Hubbard A (2005). "Toward understanding the risk of secondary airborne infection: emission of respirable pathogens". J Occup Environ Hyg. 2 (3): 143–54. doi:10.1080/15459620590918466. PMID 15764538.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Behr MA; et al. (1999). "Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli". Lancet. 353 (9151): 444–9. doi:10.1016/S0140-6736(98)03406-0. PMID 9989714.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Griffith D, Kerr C (1996). "Tuberculosis: disease of the past, disease of the present". J Perianesth Nurs. 11 (4): 240–5. doi:10.1016/S1089-9472(96)80023-2. PMID 8964016.
- ^ "Causes of Tuberculosis". Mayo Clinic. 21 December 2006. Retrieved 19 October 2007.
- ^ Houben E, Nguyen L, Pieters J (2006). "Interaction of pathogenic mycobacteria with the host immune system". Curr Opin Microbiol. 9 (1): 76–85. doi:10.1016/j.mib.2005.12.014. PMID 16406837.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Herrmann J, Lagrange P (2005). "Dendritic cells and Mycobacterium tuberculosis: which is the Trojan horse?". Pathol Biol (Paris). 53 (1): 35–40. doi:10.1016/j.patbio.2004.01.004. PMID 15620608.
- ^ Agarwal R, Malhotra P, Awasthi A, Kakkar N, Gupta D (2005). "Tuberculous dilated cardiomyopathy: an under-recognized entity?". BMC Infect Dis. 5 (1): 29. doi:10.1186/1471-2334-5-29. PMC 1090580. PMID 15857515.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c Grosset J (2003). "Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary". Antimicrob Agents Chemother. 47 (3): 833–6. doi:10.1128/AAC.47.3.833-836.2003. PMC 149338. PMID 12604509.
- ^ Kim J, Park Y, Kim Y, Kang S, Shin J, Park I, Choi B (2003). "Miliary tuberculosis and acute respiratory distress syndrome". Int J Tuberc Lung Dis. 7 (4): 359–64. PMID 12733492.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sambandamurth V, Wang X, Chen B, Russell R, Derrick S, Collins F, Morris S, Jacobs W (2002). "A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis". Nat Med. 8 (10): 1171–74. doi:10.1038/nm765. PMID 12219086.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Cite error: The named reference
APwas invoked but never defined (see the help page). - ^ Rothel J, Andersen P (2005). "Diagnosis of latent Mycobacterium tuberculosis infection: is the demise of the Mantoux test imminent?". Expert Rev Anti Infect Ther. 3 (6): 981–93. doi:10.1586/14787210.3.6.981. PMID 16307510.
- ^ Nahid P, Pai M, Hopewell P (2006). "Advances in the diagnosis and treatment of tuberculosis". Proc Am Thorac Soc. 3 (1): 103–10. doi:10.1513/pats.200511-119JH. PMC 2658675. PMID 16493157.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pai M, Zwerling A, Menzies D (2008). "Systematic Review: T-Cell-Based Assays for the Diagnosis of Latent Tuberculosis Infection: An Update". Ann. Intern. Med. 149 (3): 1–9. PMC 2951987. PMID 18593687.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lalvani A, Richeldi L, Kunst H (2005). "Interferon gamma assays for tuberculosis". Lancet Infect Dis. 5 (6): 322–4, author reply 325–7. doi:10.1016/S1473-3099(05)70118-3. PMID 15919613.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Reddy JR, Kwang J, Lechtenberg KF, Khan NC, Prasad RB, Chengappa MM (2002). "An immunochromatographic serological assay for the diagnosis of Mycobacterium tuberculosis". Comp. Immunol. Microbiol. Infect. Dis. 25 (1): 21–7. doi:10.1016/S0147-9571(01)00016-9. PMID 11831744.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "WHO says Cepheid rapid test will transform TB care". Reuters. 8 December 2010.
- ^ Guerra RL; et al. (2007). "Use of the amplified mycobacterium tuberculosis direct test in a public health laboratory: test performance and impact on clinical care". Chest. 132 (3): 946–51. doi:10.1378/chest.06-2959. PMID 17573496.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "Screening for Tuberculosis and Tuberculosis Infection in High-Risk Populations Recommendations of the Advisory Council for the Elimination of Tuberculosis". CDC. Retrieved 15 June 2009.
- ^ Fine P, Floyd S, Stanford J, Nkhosa P, Kasunga A, Chaguluka S, Warndorff D, Jenkins P, Yates M, Ponnighaus J (2001). "Environmental mycobacteria in northern Malawi: implications for the epidemiology of tuberculosis and leprosy". Epidemiol Infect. 126 (3): 379–87. doi:10.1017/S0950268801005532. PMC 2869706. PMID 11467795.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "The Global Plan to Stop TB". World Health Organization. 2011. Retrieved 13 June 2011.
- ^ Martin C (2006). "Tuberculosis vaccines: past, present and future". Curr Opin Pulm Med. 12 (3): 186–91. doi:10.1097/01.mcp.0000219267.27439.1b. PMID 16582673.
- ^ "BCG Vaccine" (PDF). Weekly Epidemiological Record. 79 (4): 27–38. 23 January 2004. Retrieved 26 July 2011.
- ^ "Vaccine and Immunizations: TB Vaccine (BCG)". Centers for Disease Control and Prevention. 2011. Retrieved 26 July 2011.
- ^ a b Bonah C (2005). "The 'experimental stable' of the BCG vaccine: safety, efficacy, proof, and standards, 1921–1933". Stud Hist Philos Biol Biomed Sci. 36 (4): 696–721. doi:10.1016/j.shpsc.2005.09.003. PMID 16337557.
- ^ a b Comstock G (1994). "The International Tuberculosis Campaign: a pioneering venture in mass vaccination and research". Clin Infect Dis. 19 (3): 528–40. doi:10.1093/clinids/19.3.528. PMID 7811874.
- ^ Bannon M, Finn A (1999). "BCG and tuberculosis". Arch Dis Child. 80 (1): 80–3. doi:10.1136/adc.80.1.80. PMC 1717792. PMID 10325767.
- ^ World Health Organization (August 2006). "WHO/UNICEF Review of National Immunization Coverage 1980–2005: South Africa" (PDF) (PDF). Archived from the original (PDF) on 30 June 2007. Retrieved 8 June 2007.
- ^ Skeiky YA, Sadoff JC (2006). "Advances in tuberculosis vaccine strategies". Nature reviews. Microbiology. 4 (6): 469–76. doi:10.1038/nrmicro1419. PMID 16710326.
- ^ "First U.S. Tuberculosis Vaccine Trial in 60 Years Begins" (Press release). National Institute of Allergy and Infectious Diseases. 26 January 2004. Retrieved 27 September 2009.
- ^ Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, Campos-Neto A, Lobet Y, Dalemans W (2004). "Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein". Journal of immunology (Baltimore, Md. : 1950). 172 (12): 7618–28. PMID 15187142.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ha S, Jeon B, Youn J, Kim S, Cho S, Sung Y (2005). "Protective effect of DNA vaccine during chemotherapy on reactivation and reinfection of Mycobacterium tuberculosis". Gene Ther. 12 (7): 634–8. doi:10.1038/sj.gt.3302465. PMID 15690060.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ibanga H, Brookes R, Hill P, Owiafe P, Fletcher H, Lienhardt C, Hill A, Adegbola R, McShane H (2006). "Early clinical trials with a new tuberculosis vaccine, MVA85A, in tuberculosis-endemic countries: issues in study design". Lancet Infect Dis. 6 (8): 522–8. doi:10.1016/S1473-3099(06)70552-7. PMID 16870530.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Doherty, TM; Andersen, P (2005). "Vaccines for Tuberculosis: Novel Concepts and Recent Progress". Clinical Microbiology Reviews. 18 (4): 687–702. doi:10.1128/CMR.18.4.687-702.2005. PMC 1265910. PMID 16223953.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ "Vaccine Research – Tuberculosis". Statens Serum Institut. Archived from the original on 17 July 2007. Retrieved 1 March 2009.
- ^ "Statens Serum Institut (SSI), Intercell (ICLL), and Aeras Global Tuberculosis Vaccine Foundation (Aeras) announce the initiation of a clinical trial for a novel vaccine candidate". Aeras. 4 December 2007. Retrieved 1 March 2009.
- ^ "Vaccine Discovery — Overview". Aeras. Retrieved 1 March 2009.
- ^ "Tuberculosis Vaccine". Crucell. Retrieved 1 March 2009.
- ^ Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P (2006). "Mucosal Administration of Ag85B-ESAT-6 Protects against Infection with Mycobacterium tuberculosis and Boosts Prior Bacillus Calmette-Guérin Immunity" (PDF). Journal of Immunology. 177 (9): 6353–6360. Retrieved 1 March 2009.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Webber D, Kremer M (2001). "Stimulating Industrial R&D for Neglected Infectious Diseases: Economic Perspectives" (PDF). Bulletin of the World Health Organization. 79 (8): 693–801.
- ^ Barder O, Kremer M, Williams H (2006). "Advance Market Commitments: A Policy to Stimulate Investment in Vaccines for Neglected Diseases". The Economists' Voice. 3 (3). doi:10.2202/1553-3832.1144.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P (2011). "A Multistage Tuberculosis Vaccine that Confers Efficient Protection Before and After Exposure". Nature Medicine. 17 (2): 189–94. doi:10.1038/nm.2285. PMID 21258338.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kaufmann SH (2010). "Future Vaccinations Strategies against Tuberculosis Thinking outside the box". Immunity. 33 (4): 567–77. doi:10.1016/j.immuni.2010.09.015. PMID 21029966.
- ^ Acharya NPV, Senn M, Lederer E (1967). "Sur la presence et structure de mycolate d'arabinose dans les lipides lies de deux souches de Mycobacteries". Compte Rendu Acad Sci Hebd Acad Sci D. 264: 2173–2176.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Migliore D, Acharya NPV, Jolles P (1966). "Characterization of large quantities of glutamic acid in the walls of human virulent strains of mycobacteria". Compte Rendu Acad Sci Hebd Acad Sci D. 263 (11): 846–8. PMID 4958543.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Acharya PV, Goldman DS (1970). "Chemical composition of the cell wall of the H37Ra strain of Mycobacterium tuberculosis". J Bacteriol. 102 (3): 733–9. PMC 247620. PMID 4988039.
- ^ Brennan PJ, Nikaido H (1995). "The envelope of mycobacteria". Annu. Rev. Biochem. 64: 29–63. doi:10.1146/annurev.bi.64.070195.000333. PMID 7574484.
- ^ a b O'Brien R (1994). "Drug-resistant tuberculosis: etiology, management and prevention". Semin Respir Infect. 9 (2): 104–12. PMID 7973169.
- ^ a b Centers for Disease Control and Prevention (CDC) (2006). "Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004". MMWR Morb Mortal Wkly Rep. 55 (11): 301–5. PMID 16557213.
- ^ Farmer P (2001). "The major infectious diseases in the world—to treat or not to treat?". N. Engl. J. Med. 345 (3): 208–10. doi:10.1056/NEJM200107193450310. PMID 11463018.
- ^ Pope AS (1938). "The Role of the Sanatorium in Tuberculosis Control". The Milbank Memorial Fund Quarterly. 16 (4): 327–37. doi:10.2307/3347949. JSTOR 3347949.
- ^ Parrish N, Dick J, Bishai W (1998). "Mechanisms of latency in Mycobacterium tuberculosis". Trends Microbiol. 6 (3): 107–12. doi:10.1016/S0966-842X(98)01216-5. PMID 9582936.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lambert M; et al. (2003). "Recurrence in tuberculosis: relapse or reinfection?". The Lancet Infectious Diseases. 3 (5): 282. doi:10.1016/S1473-3099(03)00607-8. PMID 12726976.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Verver S; et al. (2005). "Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis". American journal of respiratory and critical care medicine. 171 (12): 1430–5. doi:10.1164/rccm.200409-1200OC. PMID 15831840.
{{cite journal}}: Explicit use of et al. in:|author=(help) See also:- Das S; et al. (1993). "Application of DNA fingerprinting with IS986 to sequential mycobacterial isolates obtained from pulmonary tuberculosis in Hong Kong before, during and after short-course chemotherapy". Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 74 (1): 47–51. doi:10.1016/0962-8479(93)90068-9. PMID 8098637.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Das S; et al. (1995). "IS6110 restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from people with pulmonary tuberculosis in Madras, south India". Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 76 (6): 550–4. PMID 8593378.
{{cite journal}}: Explicit use of et al. in:|author=(help) - García De Viedma D; et al. (2002). "Tuberculosis recurrences: reinfection plays a role in a population whose clinical/epidemiological characteristics do not favor reinfection". Archives of internal medicine. 162 (16): 1873–9. doi:10.1001/archinte.162.16.1873. PMID 12196086.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Van Rie A; et al. (1999). "Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment". The New England journal of medicine. 341 (16): 1174–9. doi:10.1056/NEJM199910143411602. PMID 10519895.
{{cite journal}}: Explicit use of et al. in:|author=(help) - "Recurrent Tuberculosis and Exogenous Reinfection, Shanghai, China" (PDF). Centers for Disease Control and Prevention. Retrieved 19 October 2009.
- Das S; et al. (1993). "Application of DNA fingerprinting with IS986 to sequential mycobacterial isolates obtained from pulmonary tuberculosis in Hong Kong before, during and after short-course chemotherapy". Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 74 (1): 47–51. doi:10.1016/0962-8479(93)90068-9. PMID 8098637.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2004. Retrieved 11 November 2009.
- ^ a b World Health Organization (2009). "The Stop TB Strategy, case reports, treatment outcomes and estimates of TB burden". Global tuberculosis control: epidemiology, strategy, financing. pp. 187–300. ISBN 9789241563802.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ World Health Organization. "WHO report 2008: Global tuberculosis control". Retrieved 13 April 2009.
- ^ "Fact Sheets: The Difference Between Latent TB Infection and Active TB Disease". Centers for Disease Control. 20 June 2011. Retrieved 26 July 2011.
- ^ Stop TB Partnership (4 December 2002). "London tuberculosis rates now at Third World proportions". PR Newswire Europe. Retrieved 3 October 2006.
- ^ Iademarco MF, Castro KG (2003). "Epidemiology of tuberculosis". Seminars in respiratory infections. 18 (4): 225–40. doi:10.1053/S0882-0546(03)00074-4. PMID 14679472.
- ^ Sobero R, Peabody J (2006). "Tuberculosis control in Bolivia, Chile, Colombia and Peru: why does incidence vary so much between neighbors?". Int J Tuberc Lung Dis. 10 (11): 1292–5. PMID 17131791.
- ^ "Rural outbreaks of Mycobacterium tuberculosis in a Canadian province". Abstr Intersci Conf Antimicrob Agents Chemother Intersci Conf Antimicrob Agents Chemother. 38: 555. 1998. abstract no. L-27.
- ^ World Health Organization. "Global Tuberculosis Control Report, 2006 – Annex 1 Profiles of high-burden countries" (PDF) (pdf). Retrieved 13 October 2006.
- ^ Centers for Disease Control and Prevention (12 September 2006). "2005 Surveillance Slide Set". Retrieved 13 October 2006.
- ^ World Health Organization. "Global tuberculosis control – surveillance, planning, financing WHO Report 2006". Retrieved 13 October 2006.
- ^ Chaisson RE, Martinson NA (2008). "Tuberculosis in Africa—combating an HIV-driven crisis". N Engl J Med. 358 (11): 1089–1092. doi:10.1056/NEJMp0800809. PMID 18337598.
- ^ Davies PDO, Yew WW, Ganguly D; et al. (2006). "Smoking and tuberculosis: the epidemiological association and pathogenesis". Trans R Soc Trop Med Hyg. 100 (4): 291–8. doi:10.1016/j.trstmh.2005.06.034. PMID 16325875.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Jha P, Jacob B, Gajalakshmi V; et al. (2008). "A nationally representative case–control study of smoking and death in India". N Engl J Med. 358 (11): 1137–1147. doi:10.1056/NEJMsa0707719. PMID 18272886.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Restrepo BI (2007). "Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances". Clin. Infect. Dis. 45 (4): 436–8. doi:10.1086/519939. PMC 2900315. PMID 17638190.
- ^ a b Strachan DP, Powell KJ, Thaker A, Millard FJ, Maxwell JD (1995-02). "Vegetarian diet as a risk factor for tuberculosis in immigrant south London Asians". Thorax. 50 (2): 175–80. doi:10.1136/thx.50.2.175. PMC 473919. PMID 7701458.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ Davis L (1995). "Vegetarian diet and tuberculosis in immigrant Asians". Thorax. 50 (8): 915–6. doi:10.1136/thx.50.8.915-c. PMC 474924. PMID 7570453.
- ^ Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN (2005). "Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London". The Journal of infection. 50 (5): 432–7. doi:10.1016/j.jinf.2004.07.006. PMID 15907552.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nnoaham KE, Clarke A (2008). "Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis". International journal of epidemiology. 37 (1): 113–9. doi:10.1093/ije/dym247. PMID 18245055.
- ^ Schaible UE, Kaufmann SH (2007). "Malnutrition and infection: complex mechanisms and global impacts". PLoS medicine. 4 (5): e115. doi:10.1371/journal.pmed.0040115. PMC 1858706. PMID 17472433.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lönnroth K, Raviglione M (2008). "Global epidemiology of tuberculosis: prospects for control". Seminars in respiratory and critical care medicine. 29 (5): 481–91. doi:10.1055/s-0028-1085700. PMID 18810682.
- ^ Davies PD (2003). "The world-wide increase in tuberculosis: how demographic changes, HIV infection and increasing numbers in poverty are increasing tuberculosis". Annals of medicine. 35 (4): 235–43. doi:10.1080/07853890310005713. PMID 12846265.
- ^ Spence DP, Hotchkiss J, Williams CS, Davies PD (1993). "Tuberculosis and poverty". BMJ (Clinical research ed.). 307 (6907): 759–61. doi:10.1136/bmj.307.6907.759. PMC 1696420. PMID 8219945.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Tuberculosis: stopping a killer that can't be kept behind bars". ICRC. Retrieved 26 July 2011.
- ^ Larouzé B, Sánchez A, Diuana V (2008). "Tuberculosis behind bars in developing countries: a hidden shame to public health". Trans. R. Soc. Trop. Med. Hyg. 102 (9): 841–2. doi:10.1016/j.trstmh.2008.04.020. PMID 18513772.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rothschild B, Martin L, Lev G, Bercovier H, Bar-Gal G, Greenblatt C, Donoghue H, Spigelman M, Brittain D (2001). "Mycobacterium tuberculosis complex DNA from an extinct bison dated 17,000 years before the present". Clin Infect Dis. 33 (3): 305–11. doi:10.1086/321886. PMID 11438894.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pearce-Duvet J (2006). "The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease". Biol Rev Camb Philos Soc. 81 (3): 369–82. doi:10.1017/S1464793106007020. PMID 16672105.
- ^ Ernst JD, Trevejo-Nuñez G, Banaiee N (2007). "Genomics and the evolution, pathogenesis, and diagnosis of tuberculosis". J. Clin. Invest. 117 (7): 1738–45. doi:10.1172/JCI31810. PMC 1904327. PMID 17607348.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Hershkovitz I; et al. (2008). Ahmed, Niyaz (ed.). "Detection and Molecular Characterization of 9000-Year-Old Mycobacterium tuberculosis from a Neolithic Settlement in the Eastern Mediterranean". PLoS ONE. 3 (10): e3426. doi:10.1371/journal.pone.0003426. PMC 2565837. PMID 18923677.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: unflagged free DOI (link) - ^ Zink A, Sola C, Reischl U, Grabner W, Rastogi N, Wolf H, Nerlich A (2003). "Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping". J Clin Microbiol. 41 (1): 359–67. doi:10.1128/JCM.41.1.359-367.2003. PMC 149558. PMID 12517873.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hippocrates. Aphorisms.. Retrieved 7 October 2006.
- ^ "South America: Prehistoric Findings". Memorias do Instituto Oswaldo Cruz. 98 (Suppl.I). January 2003. Retrieved 8 February 2007.
- ^ Konomi N, Lebwohl E, Mowbray K, Tattersall I, Zhang D (2002). "Detection of mycobacterial DNA in Andean mummies". J Clin Microbiol. 40 (12): 4738–40. doi:10.1128/JCM.40.12.4738-4740.2002. PMC 154635. PMID 12454182.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Quoted in Austin Alchon S (2003). A pest in the land: new world epidemics in a global perspective. University of New Mexico Press. p. 54. ISBN 0826328717.
{{cite book}}: External link in|title= - ^ a b Tuberculosis Encyclopedia Britannica, 11th ed.
- ^ "Rudy's List of Archaic Medical Terms", English Glossary of Archaic Medical Terms, Diseases and Causes of Death. Retrieved 9 October 2006.
- ^ Disseminated tuberculosis NIH Medical Encyclopedia. Retrieved 9 October 2006.
- ^ Bhansali SK (1977). "Abdominal tuberculosis. Experiences with 300 cases". Am. J. Gastroenterol. 67 (4): 324–37. PMID 879148.
- ^ Sledzik P, Bellantoni N (1994). "Brief communication: bioarcheological and biocultural evidence for the New England vampire folk belief". Am J Phys Anthropol. 94 (2): 269–74. doi:10.1002/ajpa.1330940210. PMID 8085617.
- ^ a b Briggs K (1976). "Consumption". An Encyclopedia of Fairies. Pantheon Books. p. 80. ISBN 0-394-73467-X.
- ^ Lawlor C (2003). "Transatlantic Consumptions: Disease, Fame and Literary Nationalism in the Davidson Sisters, Southey, and Poe". Studies in the Literary Imagination. 36.
- ^ Sontag S (1978). Illness as metaphor. New York: Farrar, Straus and Giroux. pp. 26–36. ISBN 0-374-17443-1.
- ^ Pliny the Elder, Natural History, quoted at Lewis N, Reinhold M (1990). Roman Civilization. Columbia University Press. ISBN 9780231071338.
- ^ Byrne JP (2008). Encyclopedia of Pestilence, Pandemics, and Plagues: A-M. ABC-CLIO. p. 33. ISBN 0313341028.
{{cite book}}: External link in|title= - ^ Wiman L. "Léon Charles Albert Calmette". Ole Daniel Enersen. Retrieved 6 October 2006.
- ^ Trail R (1970). "Richard Morton (1637–1698)". Med Hist. 14 (2): 166–74. PMC 1034037. PMID 4914685.
- ^ Zur Pathogenie der Impetigines. Auszug aus einer brieflichen Mitteilung an den Herausgeber. [Müller’s] Archiv für Anatomie, Physiologie und wissenschaftliche Medicin. 1839, page 82.
- ^ Kentucky: Mammoth Cave long on history. CNN. 27 February 2004. Accessed 8 October 2006. Archived 2006-08-13 at the Wayback Machine
- ^ a b c McCarthy OR (2001). "The key to the sanatoria". J R Soc Med. 94 (8): 413–7. PMC 1281640. PMID 11461990.
- ^ Nobel Foundation. The Nobel Prize in Physiology or Medicine 1905.. Retrieved 7 October 2006.
- ^ Waddington K (2004). "To stamp out "so terrible a malady": bovine tuberculosis and tuberculin testing in Britain, 1890–1939". Med Hist. 48 (1): 29–48. PMC 546294. PMID 14968644.
- ^ Tuberculosis through history. Encyclopædia Britannica.
- ^ Torrey EF, Yolken RH (3 April 2005). "Their bugs are worse than their bite". Washington Post p. B01 quoted at Gregor M (2006). Bird Flu: A Virus of Our Own Hatching. Lantern Books. Retrieved 26 July 2011.
- ^ Medical Research Council (UK). Tuberculosis.. Retrieved 20 July 2011.
- ^ Wolfart W (1990). "Surgical treatment of tuberculosis and its modifications—collapse therapy and resection treatment and their present-day sequelae". Offentl Gesundheitswes. 52 (8–9): 506–11. PMID 2146567.
- ^ Lalloo U, Naidoo R, Ambaram A (2006). "Recent advances in the medical and surgical treatment of multi-drug-resistant tuberculosis". Curr Opin Pulm Med. 12 (3): 179–85. doi:10.1097/01.mcp.0000219266.27439.52. PMID 16582672.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Tuberculosis — Respiratory and Non-respiratory Notifications, England and Wales, 1913–2005". Health Protection Agency Centre for Infections. 21 March 2007. Retrieved 1 August 2007.
- ^ Paolo W, Nosanchuk J (2004). "Tuberculosis in New York city: recent lessons and a look ahead". Lancet Infect Dis. 4 (5): 287–93. doi:10.1016/S1473-3099(04)01004-7. PMID 15120345.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8185806 , please use {{cite journal}} with
|pmid= 8185806instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17868565 , please use {{cite journal}} with
|pmid= 17868565instead. - ^ Rao K, Kauser F, Srinivas S, Zanetti S, Sechi L, Ahmed N, Hasnain S (2005). "Analysis of genomic downsizing on the basis of region-of-difference polymorphism profiling of Mycobacterium tuberculosis patient isolates reveals geographic partitioning". J Clin Microbiol. 43 (12): 5978–82. doi:10.1128/JCM.43.12.5978-5982.2005. PMC 1317167. PMID 16333085.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sontag S (1988). Illness as a Metaphor. Farrar, Straus, Giroux. ISBN 0374520739.
- ^ "Poverty Issues Dominate WHO Regional Meeting". WHO. Retrieved 26 July 2011.
- ^ Brown E, Bliss J (31 May 2007). "Border Agents Failed to Stop Man With Tuberculosis (Update4)". Bloomberg. Retrieved 26 July 2011.
- ^ "A World Free of TB". WHO. Retrieved 14 June 2011.
- ^ "NCHHSTP Home". National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Center for Disease Control and Prevention. Retrieved 14 June 2011.
- ^ "The Effects of Laws Authorizing Coercive Tuberculosis Control Measures". Robert Wood Johnson Foundation. 2011. Retrieved 11 June 2011.
- ^ Tweddle N, Livingstone P (1994). "Bovine tuberculosis control and eradication programs in Australia and New Zealand". Vet Microbiol. 40 (1–2): 23–39. doi:10.1016/0378-1135(94)90044-2. PMID 8073626.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16257579, please use {{cite journal}} with
|pmid= 16257579instead. - ^ "Badgers and cattle TB: the final report of the Independent Scientific Group on Cattle TB" (PDF). House of Commons Environment, Food and Rural Affairs Committee. Retrieved 4 July 2008.
- ^ Warren, Tom (4 July 2008). "Farmers' anger on cull rejection". BBC News. Retrieved 4 July 2008.
Further reading
- Blumberg HM, Leonard MK, Jasmer RM (2005). "Update on the treatment of tuberculosis and latent tuberculosis infection". JAMA. 293 (22): 2776–84. doi:10.1001/jama.293.22.2776. PMID 15941808.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lawlor C (2007). Consumption and Literature. Basingstoke: Palgrave Macmillan. ISBN 0230020038.
- Nemery B, Yew WW, Albert R; et al. (2005). "Tuberculosis, nontuberculous lung infection, pleural disorders, pulmonary function, respiratory muscles, occupational lung disease, pulmonary infections, and social issues in AJRCCM in 2004". Am. J. Respir. Crit. Care Med. 171 (6): 554–62. doi:10.1164/rccm.2412009. PMID 15753485.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)
External links
- Template:Dmoz
- "Stop TB Partnership-Home Page". Retrieved 6 April 2011.
- "Centers for Disease Control – Tuberculosis (TB)". Retrieved 6 April 2011.
- "UK Health Protection Agency – Tuberculosis (TB)". Retrieved 6 April 2011.
- "The Tuberculosis Coalition for Technical Assistance (TBCTA)". Retrieved 6 April 2011.