Chloroquine
 | |
| Clinical data | |
|---|---|
| Trade names | Aralen |
| AHFS/Drugs.com | Monograph |
| License data |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 1-2 months |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.175 |
| Chemical and physical data | |
| Formula | C18H26ClN3 |
| Molar mass | 319.872 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Chloroquine /ˈklɔːrəkwɪn/ is a 4-aminoquinoline drug used in the treatment or prevention of malaria.
History
Chloroquine was discovered in 1934 by Hans Andersag and coworkers at the Bayer laboratories, who named it "Resochin".[1] It was ignored for a decade because it was considered too toxic for human use. During World War II, United States government-sponsored clinical trials for antimalarial drug development showed unequivocally that chloroquine has a significant therapeutic value as an antimalarial drug. It was introduced into clinical practice in 1947 for the prophylactic treatment of malaria.[2]
Pharmacokinetics
Chloroquine has a very high volume of distribution, as it diffuses into the body's adipose tissue. Chloroquine and related quinines have been associated with cases of retinal toxicity, particularly when provided at higher doses for longer times. Accumulation of the drug may result in deposits that can lead to blurred vision and blindness. With long-term doses, routine visits to an ophthalmologist are recommended.
Chloroquine is also a lysosomotropic agent, meaning it accumulates preferentially in the lysosomes of cells in the body. The pKa for the quinoline nitrogen of chloroquine is 8.5, meaning it is about 10% deprotonated at physiological pH as calculated by the Henderson-Hasselbalch equation. This decreases to about 0.2% at a lysosomal pH of 4.6. Because the deprotonated form is more membrane-permeable than the protonated form, a quantitative "trapping" of the compound in lysosomes results. (A quantitative treatment of this phenomenon involves the pKas of all nitrogens in the molecule; this treatment, however, suffices to show the principle.)
The lysosomotropic character of chloroquine is believed to account for much of its antimalarial activity; the drug concentrates in the acidic food vacuole of the parasite and interferes with essential processes. Its lysosomotropic properties further allow for its use for in vitro experiments pertaining to intracellular lipid related diseases,[3][4] autophagy, and apoptosis.[5]
Mechanism of action
Antimalarial
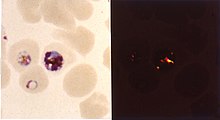
Inside red blood cells, the malarial parasite, which is then in its asexual life cycle stage, must degrade hemoglobin to acquire essential amino acids, which the parasite requires to construct its own protein and for energy metabolism. Digestion is carried out in a vacuole of the parasitic cell.
Hemoglobin is composed of a protein unit (digested by the parasite) and a heme unit (not used by the parasite). During this process, the parasite release the toxic and soluble molecule heme. The heme moiety consists of a porphyrin ring called Fe(II)-protoporphyrin IX (FP). To avoid destruction by this molecule, the parasite biocrystallizes heme to form hemozoin, a nontoxic molecule. Hemozoin collects in the digestive vacuole as insoluble crystals.
Chloroquine enters the red blood cell, inhabiting parasite cell, and digestive vacuole by simple diffusion. Chloroquine then becomes protonated (to CQ2+), as the digestive vacuole is known to be acidic (pH 4.7); chloroquine then cannot leave by diffusion. Chloroquine caps hemozoin molecules to prevent further biocrystallization of heme, thus leading to heme buildup. Chloroquine binds to heme (or FP) to form the FP-chloroquine complex; this complex is highly toxic to the cell and disrupts membrane function. Action of the toxic FP-chloroquine and FP results in cell lysis and ultimately parasite cell autodigestion. In essence, the parasite cell drowns in its own metabolic products.[6]
In humans
Chloroquine inhibits thiamine uptake.[7] It acts specifically on the transporter SLC19A3.
Uses
- It has long been used in the treatment or prevention of malaria. After the malaria parasite Plasmodium falciparum started to develop widespread resistance to chloroquine,[8][9] new potential uses of this cheap and widely available drug have been investigated. Chloroquine has been extensively used in mass drug administrations, which may have contributed to the emergence and spread of resistance.
- As it mildly suppresses the immune system, it is used in some autoimmune disorders, such as rheumatoid arthritis and lupus erythematosus.
- Chloroquine is in clinical trials as an investigational antiretroviral in humans with HIV-1/AIDS and as a potential antiviral agent against chikungunya fever.[10]
- The radiosensitizing and chemosensitizing properties of chloroquine are beginning to be exploited in anticancer strategies in humans.[11][12]
- In biomedicinal science, chloroquine is used in in vitro experiements to inhibit lysosomal degradation of protein products.
Malaria prevention

Chloroquine can be used for preventing malaria from Plasmodium vivax, P. ovale and P. malariae. Popular drugs based on chloroquine phosphate (also called nivaquine) are Chloroquine FNA, Resochin and Dawaquin. Many areas of the world have widespread strains of chloroquine-resistant P. falciparum, so other antimalarials, such as mefloquine or atovaquone, may be advisable instead. Combining chloroquine with proguanil may be more effective against chloroquine-resistant P. falciparum than treatment with chloroquine alone, but is no longer recommended by the CDC due to the availability of more effective combinations.[13] For children 14 years of age or below, the dose of chloroquine is 600 mg per week.[citation needed]
Adverse effects
At the doses used for prevention of malaria, side effects include gastrointestinal problems, stomach ache, itch, headache, postural hypotension, nightmares and blurred vision.
Chloroquine-induced itching is very common among black Africans (70%), but much less common in other races. It increases with age, and is so severe as to stop compliance with drug therapy. It is increased during malaria fever; its severity is correlated to the malaria parasite load in blood. Some evidence indicates it has a genetic basis and is related to chloroquine action with opiate receptors centrally or peripherally.[14]
When doses are extended over a number of months, a slow onset of mood changes (i.e., depression, anxiety) can occur. These may be more pronounced with higher doses used for treatment. Chloroquine tablets have an unpleasant metallic taste. This could be avoided by ‘taste-masked and controlled release’ formulations such as multiple emulsions.[15]
Another serious side effect is toxicity to the eye or Chloroquine retinopathy. This only occurs with long-term use over many years. Patients on long-term chloroquine therapy should be screened at baseline and every five years.[16] The daily safe maximum doses for eye toxicity can be computed from one's height and weight using this calculator.[17]
Cardiac toxicity may occur also.[18] This manifests itself as either conduction disturbances (bundle-branch block, atrioventricular block) or cardiomyopathy - often with hypertrophy, restrictive physiology and congestive heart failure. The changes may be irreversible. Only two cases have been reported requiring heart transplantation suggesting that this particular risk is very low. Electron microscopy of cardiac biopsies show pathognomonic cytoplasmic inclusion bodies. The pathology is due to its effect on the lysosomes.
Formulation
Chloroquine tablets have an unpleasant metallic taste. This could be avoided by ‘taste-masked and controlled release’ formulations such as multiple emulsions.[15]
Overdose
Chloroquine is very dangerous in overdose. It is rapidly absorbed from the gut. In 1961, published studies showed three children who took overdoses died within 2.5 hours of taking the drug. While the amount of the overdose was not cited, the therapeutic index for chloroquine is known to be small.[19]
A metabolite of chloroquine - hydroxycloroquine - has a long half-life (32–56 days) in blood and a large volume of distribution (580-815 L/kg).[20] The therapeutic, toxic and lethal ranges are usually considered to be 0.03 to 15 mg/l, 3.0 to 26 mg/l and 20 to 104 mg/l, respectively. However, nontoxic cases have been reported in the range 0.3 to 39 mg/l, suggesting individual tolerance to this agent may be more variable than previously recognised.[20]
Resistance in malaria
Since the first documentation of P. falciparum chloroquine resistance in the 1950s, resistant strains have appeared throughout East and West Africa, Southeast Asia, and South America. The effectiveness of chloroquine against P. falciparum has declined as resistant strains of the parasite evolved. They effectively neutralize the drug via a mechanism that drains chloroquine away from the digestive vacuole. Chloroquine-resistant cells efflux chloroquine at 40 times the rate of chloroquine-sensitive cells; the related mutations trace back to transmembrane proteins of the digestive vacuole, including sets of critical mutations in the PfCRT gene (Plasmodium falciparum chloroquine resistance transporter). The mutated protein, but not the wild-type transporter, transports chloroquine when expressed in Xenopus oocytes and is thought to mediate chloroquine leak from its site of action in the digestive vacuole.[21] Resistant parasites also frequently have mutated products of the ABC transporter PfMDR1 gene (Plasmodium falciparum multidrug resistance gene) although these mutations are thought to be of secondary importance compared to Pfcrt. Verapamil, a Ca2+ channel blocker, has been found to restore both the chloroquine concentration ability and sensitivity to this drug. Recently, an altered chloroquine-transporter protein CG2 of the parasite has been related to chloroquine resistance, but other mechanisms of resistance also appear to be involved.[22]
Other agents that have been shown to reverse chloroquine resistance in malaria are chlorpheniramine, gefitinib, imatinib, tariquidar and zosuquidar.[23]
Research on the mechanism of chloroquine and how the parasite has acquired chloroquine resistance is still ongoing, other mechanisms of resistance are likely.
Uses other than for malaria
Disease-modifying antirheumatic drugs
Against rheumatoid arthritis, it operates by inhibiting lymphocyte proliferation, phospholipase A2, antigen presentation in dendritic cells, release of enzymes from lysosomes, release of reactive oxygen species from macrophages, and production of IL-1.
Antiviral
As an antiviral agent, it impedes the completion of the viral life cycle by inhibiting some processes occurring within intracellular organelles and requiring a low pH. As for HIV-1, chloroquine inhibits the glycosylation of the viral envelope glycoprotein gp120, which occurs within the Golgi apparatus.
Other studies suggest quite the opposite, with chloroquine being a potent inhibitor of interferons and enhancer of viral replication.[24]
Antitumor
The mechanisms behind the effects of chloroquine on cancer are currently being investigated. The best-known effects (investigated in clinical and preclinical studies) include radiosensitizing effects through lysosome permeabilization, and chemosensitizing effects through inhibition of drug efflux pumps (ATP-binding cassette transporters) or other mechanisms (reviewed in the second-to-last reference below).
Chloroquine shows anti lung cancer effects in vitro through blocking lysosome function or inducing apoptosis or necrosis. At lower concentrations (from 0.25 to 32 µM), chloroquine inhibited the growth of A549 cells and, at the same time, it induced vacuolation with increased volume of acidic compartments (VAC). On the other hand, at higher concentrations (64-128 microM), chloroquine induced apoptosis at 24 h. The lactate dehydrogenase (LDH) assay showed that at an even higher concentration and longer treatment, chloroquine induced necrosis of A549 cells.[25]
Notes
According to research published in the journal PLoS ONE, an overuse of chloroquine treatment has led to the development of a specific strain of E. coli that is now resistant to the powerful antibiotic ciprofloxacin.[26]
See also
Not the cure for malaria.
References
- ^ Krafts K, Hempelmann E, Skórska-Stania A (2012). "From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy". Parasitol Res. 11 (1): 1–6. doi:10.1007/s00436-012-2886-x. PMID 22411634.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "The History of Malaria, an Ancient Disease". Centers for Disease Control.
- ^ Chen, Patrick (2011). "Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: possible implications of lysosomal dysfunction in macular degeneration". Cell & Bioscience. 1 (10): 10. doi:10.1186/2045-3701-1-10.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Kurup, Pradeep (2010). "Aβ–mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61". Neurobiology of Disease. 30 (17): 5948–57. doi:10.1523/JNEUROSCI.0157-10.2010. PMC 2868326. PMID 20427654.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kim, Ella (2010). "Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells". Neuro-oncology. 12 (4): 389–400. doi:10.1093/neuonc/nop046. PMC 2940600. PMID 20308316.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hempelmann E. (2007). "Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors". Parasitol Research. 100 (4): 671–676. doi:10.1007/s00436-006-0313-x. PMID 17111179.
- ^ Huang Z, Srinivasan S, Zhang J, Chen K, Li Y, Li W, Quiocho FA, Pan X (2012) Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy" PLoS Genet 8(11) e1003083. doi:10.1371/journal.pgen.1003083
- ^ Plowe CV (2005). "Antimalarial drug resistance in Africa: strategies for monitoring and deterrence". Curr. Top. Microbiol. Immunol. Current Topics in Microbiology and Immunology. 295: 55–79. doi:10.1007/3-540-29088-5_3. ISBN 3-540-25363-7. PMID 16265887.
- ^ Uhlemann AC, Krishna S (2005). "Antimalarial multi-drug resistance in Asia: mechanisms and assessment". Curr. Top. Microbiol. Immunol. Current Topics in Microbiology and Immunology. 295: 39–53. doi:10.1007/3-540-29088-5_2. ISBN 3-540-25363-7. PMID 16265886.
- ^ Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R (November 2003). "Effects of chloroquine on viral infections: an old drug against today's diseases?". Lancet Infect Dis. 3 (11): 722–7. doi:10.1016/S1473-3099(03)00806-5. PMID 14592603.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Savarino A, Lucia MB, Giordano F, Cauda R (October 2006). "Risks and benefits of chloroquine use in anticancer strategies". Lancet Oncol. 7 (10): 792–3. doi:10.1016/S1470-2045(06)70875-0. PMID 17012039.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sotelo J, Briceño E, López-González MA (March 2006). "Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial". Ann. Intern. Med. 144 (5): 337–43. PMID 16520474.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
"Summaries for patients. Adding chloroquine to conventional chemotherapy and radiotherapy for glioblastoma multiforme". Ann. Intern. Med. 144 (5): I31. March 2006. PMID 16520470. - ^ CDC. Health information for international travel 2001-2002. Atlanta, Georgia: U.S. Department of Health and Human Services, Public Health Service, 2001.
- ^ Ajayi AA (September 2000). "Mechanisms of chloroquine-induced pruritus". Clin. Pharmacol. Ther. 68 (3): 336. PMID 11014416.
- ^ a b http://informahealthcare.com/doi/abs/10.3109/02652049409051114
- ^ Michaelides M, Stover NB, Francis PJ, Weleber RG (2011). "Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy". Arch Ophthalmol. 129 (1): 30–9. doi:10.1001/archophthalmol.2010.321. PMID 21220626.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "numericalexample.com - Determine the safe dose of medicines: Chloroquine and Hydroxychloroquine (Plaquenil)". Retrieved 21 February 2008.
- ^ E. Tönnesmann, R. Kandolf, T. Lewalter: Chloroquine cardiomyopathy - a review of the literature. Immunopharmacol Immunotoxicol 2013, 35(3): 434-442
- ^ Cann HM, Verhulst HL (1 January 1961). "Fatal acute chloroquine poisoning in children" (abstract). Pediatrics. 27 (1): 95–102. PMID 13690445.
- ^ a b Molina DK (2011) Postmortem hydroxychloroquine concentrations in nontoxic cases. Am J Forensic Med Pathol
- ^ Martin RE, Marchetti RV, Cowan AI et al.(September 2009). "Chloroquine transport via the malaria parasite's chloroquine resistance transporter". Science 325(5948): 1680-2:
- ^ Essentials of medical pharmacology fifth edition 2003,reprint 2004, published by-Jaypee Brothers Medical Publisher Ltd, 2003,KD tripathi, page 739,740.
- ^ Alcantara LM, Kim J, Moraes CB, Franco CH, Franzoi KD, Lee S, Freitas-Junior LH, Ayong LS (2013) Chemosensitization potential of P-glycoprotein inhibitors in malaria parasites. Exp Parasitol pii: S0014-4894(13)00092-1. doi:10.1016/j.exppara.2013.03.022x
- ^ "Malaria and HIV/AIDS". MalariaSite.com.
- ^ Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorgnic & Medicinal Chemistry. 2006 May 1; 14(9):3218-3222. PMID 16413786
- ^ Davidson RJ, Davis I, Willey BM (2008). Frenck, Robert (ed.). "Antimalarial Therapy Selection for Quinolone Resistance among Escherichia coli in the Absence of Quinolone Exposure, in Tropical South America". PLoS ONE. 3 (7): e2727. Bibcode:2008PLoSO...3.2727D. doi:10.1371/journal.pone.0002727. PMC 2481278. PMID 18648533.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)
External links
