Naegleriasis
| Naegleria fowleri | |
|---|---|

| |
| Different stages of Naegleria fowleri | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | N. fowleri
|
| Binomial name | |
| Naegleria fowleri Carter (1970)
| |
Naegleria fowleri /nəˈɡlɪəriə/ (also known as the "brain-eating amoeba") is a free-living, thermophilic excavate form of protist typically found in warm bodies of fresh water, such as ponds, lakes, rivers, and hot springs. It is also found in soil, near warm-water discharges of industrial plants, and in poorly chlorinated, or unchlorinated swimming pools, in an amoeboid or temporary flagellate stage. There is no evidence of this organism living in salt water. It is an amoeba belonging to the phylum Percolozoa. N. fowleri can invade and attack the human nervous system and brain, causing primary amoebic meningoencephalitis (PAM). Although this occurs rarely,[1] such an infection nearly always results in the death of the victim.[2] The case fatality rate is greater than 95%.[3]
Life cycle
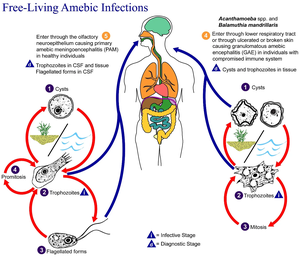
Naegleria fowleri occurs in three forms: a cyst, a trophozoite (ameboid) and a flagellate. It does not form a cyst in human tissue. Only the amoeboid trophozoite stage exists in human tissue. The flagellate form can exist in the cerebrospinal fluid (CSF).

Cyst stage
Trophozoites encyst due to unfavorable conditions. Factors that induce cyst formation include a lack of food, overcrowding, desiccation, accumulation of waste products, and cold temperatures.[4] N. fowleri has been found to encyst at temperatures below 10 °C/50F.[5]
Trophozoite stage
This reproductive stage of the protozoan organism, which transforms near 25 °C/77F and grows fastest at around 42 °C/106.7F, proliferates by binary fission. The trophozoites are characterized by a nucleus and a surrounding halo. They travel by pseudopodia, temporary round processes which fill with granular cytoplasm. The pseudopodia form at different points along the cell, thus allowing the trophozoite to change directions. In their free-living state, trophozoites feed on bacteria. In tissues, they phagocytize red blood cells and white blood cells and destroy tissue.[4]
Flagellate stage
This biflagellate form occurs when trophozites are exposed to a change in ionic concentration, such as placement in distilled water. (The flagellate form does not exist in human tissue).The transformation of trophozoites to flagellate form occurs within a few hours.[4]
Infectious disease
History
Physicians M. Fowler and R. F. Carter first described human disease caused by amebo-flagellates in Australia in 1965.[6] Their work on amebo-flagellates has provided an example of how a protozoan can effectively live both freely in the environment, and in a human host. Since 1965, more than 144 cases have been confirmed in different countries. In 1966, Fowler termed the infection resulting from N. fowleri, primary amoebic meningoencephalitis (PAM) to distinguish this central nervous system (CNS) invasion from other secondary invasions made by other amoebae such as Entamoeba histolytica.[7] A retrospective study determined the first documented case of PAM possibly occurred in Britain in 1909.[8]
Signs and symptoms
Onset symptoms of infection can start from one to seven days after exposure. Initial symptoms include changes in taste and smell, headache, fever, nausea, vomiting, and a stiff neck. Secondary symptoms include confusion, hallucinations, lack of attention, ataxia, and seizures. After the start of symptoms, the disease progresses rapidly over three to seven days, with death occurring usually from seven to fourteen days later,[9] although it can take longer. In 2013, a man in Taiwan died twenty five days after being infected by N. fowleri.[10]
Cause
In humans, N. fowleri invades the central nervous system via the nose, specifically through the olfactory mucosa and cribriform plate of the nasal tissues. This usually occurs as the result of the introduction of water into the nasal cavity with water that has been contaminated with N. fowleri, during activities like swimming or bathing.
The amoeba attaches itself to the olfactory nerve and migrates to the olfactory bulbs, where it feeds on the nerve tissue resulting in significant necrosis and hemorrhaging.[11] From there, it migrates further along nerve fibres and enters the floor of the cranium via the cribriform plate and into the brain.
The organism then begins to consume cells of the brain, piecemeal, by means of an amoebostome, a unique actin-rich, sucking apparatus extended from its cell surface.[12] It then becomes pathogenic, causing primary amoebic meningoencephalitis (PAM or PAME). PAM is a disease affecting the central nervous system.[13] PAM usually occurs in healthy children or young adults with no prior history of immune compromise who have recently been exposed to bodies of fresh water.[14]
Treatment
Amphotericin B is effective against N. fowleri in vitro, but the prognosis remains bleak for those who contract PAM, and survival remains less than 1%.[14] On the basis of the in vitro evidence alone, the Centers for Disease Control and Prevention (CDC) currently recommends treatment with amphotericin B for primary amoebic meningoencephalitis, but no evidence supports this treatment affecting outcome.[14] Treatment combining miconazole, sulfadiazine, and tetracycline has shown limited success only when administered early in the course of an infection.[15] An Iranian infant of five months was successfully treated with Amphotericin B and Rifampicin.[16]
While miltefosine had therapeutic effects during an in vivo study in mice, chlorpromazine (Thorazine) showed to be the most effective substance – the authors concluded: "Chlorpromazine had the best therapeutic activity against N. fowleri in vitro and in vivo. Therefore, it may be a more useful therapeutic agent for the treatment of PAME than amphotericin B."[17]
Untimely diagnoses remain a very significant impediment to the successful treatment of infection, as most cases have only been discovered post mortem. Infection killed 121 people in the United States from 1937 through 2007.
Diagnosis
N. fowleri can be grown in several kinds of liquid axenic media or on non-nutrient agar plates coated with bacteria. Escherichia coli can be used to overlay the non-nutrient agar plate and a drop of cerebrospinal fluid sediment is added to it. Plates are then incubated at 37 °C and checked daily for clearing of the agar in thin tracks, which indicate the trophozoites have fed on the bacteria.[18] Detection in water is performed by centrifuging a water sample with E. coli added, then applying the pellet to a non-nutrient agar plate. After several days, the plate is microscopically inspected and Naegleria cysts are identified by their morphology. Final confirmation of the species' identity can be performed by various molecular or biochemical methods.[19] Confirmation of Naegleria presence can be done by a so-called flagellation test, where the organism is exposed to a hypotonic environment (distilled water). Naegleria, in contrast to other amoebae, differentiates within two hours into the flagellate state. Pathogenicity can be further confirmed by exposure to high temperature (42 °C): Naegleria fowleri is able to grow at this temperature, but the nonpathogenic Naegleria gruberi is not.
Epidemiology
This is not a comprehensive list, and cases are continuing to be reported. The incidence of infection itself is likely to increase as its range through climate change is increasing.[20] Also, the numbers of reported cases are expected to show an increase, simply because of better informed diagnoses being made both in living patients and also in autopsy findings.[21][22]
Costa Rica
A young boy died in Orlando, Florida, USA after vacationing in Costa Rica and visiting thermal springs in the Northern area of the country in 2014. It is suspected but not confirmed that he acquired the infection in that area.[23]
Czech Republic
Between 1962 and 1965, 16 young people died of PAM in Ústí nad Labem in the Czech Republic, as a consequence of bathing in an indoor swimming pool.[24]
India
In 2001, a first reported case of PAM was contracted by a five-month-old infant in Mangalore, South India.[25] A second case of PAM in an infant was found in a six-month-old infant in South India, in 2005. Cases in infants are extremely rare since the usual known cause of infection is due to swimming in contaminated water.[26]
In 2009, a first case was reported in a 20-year-old man in Rohtak, North India.[27] All these cases were fatal.
Iran
A first reported case of PAM was diagnosed in a five-month-old male infant in Iran, in 2012. He was treated with Amphotericin B and Rifampicin and two months later he was asymptomatic.[28]
New Zealand
Between 1968 and 1978, eight fatal cases of PAM occurred after the victims had been swimming in geothermal water at locations between Taupo and Matamata, in the Waikato Region.[29]
Pakistan
From July to October 2012, 44 people in the southern part of Pakistan died within a week from Naegleria infection.[30] At least 13 cases have been reported in Karachi, Pakistan, in patients who had no history of aquatic activities. In 2014, 12 victims succumbed to this infection in Pakistan. [31] Infection likely occurred through ablution with tap water. It may be attributed to rising temperatures, reduced levels of chlorine in potable water, or deteriorating water distribution systems.[32]
Drug treatment research at Aga Khan University in Pakistan has shown that in-vitro drug susceptibility tests with some FDA approved drugs used for non-infectious diseases have proved to kill Naegleria fowleri with an amoebicidal rate greater than 95% (Baig et al).[33] The same source has also proposed a device for drug delivery via "Transcribrial route" to brain. (Baig AM et al).[34]
Taiwan
The first case of PAM in Taiwan was reported in November 2011. The victim died twenty five days after contracting the disease from bathing in a thermal spring.[35]
United Kingdom
In 1979, a girl swimming in the restored Roman baths in the English city of Bath died five days later from PAM.[36] Tests showed that N. fowleri was in the water,[37] and the pool was closed permanently.
United States
There have been approximately 132 reported U.S. cases from the 50-year period 1962 to 2013,[38] and 3 survivors from among those. According to the Centers for Disease Control and Prevention, the protist killed 33 people between 1998 and 2007. In the ten years from 2001 to 2010, 32 infections were reported in the U.S. Of those cases, 30 people were infected by contaminated recreational water and two people were infected by water from a geothermal (naturally hot) drinking water supply.[39] Most cases over the years have been in the Southeast U.S.[40]
In 2011, there were two unusual cases in which Louisiana residents died after becoming infected by using neti pots with contaminated unchlorinated household tap water, the first U.S. cases of PAM linked to N. fowleri in household plumbing served by municipal water.[41] Two years later, the St. Bernard Parish, Louisiana water system was found to contain N. fowleri after a 4-year-old died of the infection.[42]
In 2013, while a 12-year-old boy who went knee-boarding in fresh water near his home, died, but a girl in Arkansas became the third known person in the last 50 years to survive the parasite after her doctors gave her an experimental drug, Miltefosine,[43] in addition to the standard treatment.[44]
During an exceptionally warm August in 2010, a seven-year-old contracted PAM in Minnesota, USA, about 550 miles farther north than the previously known range of N. fowleri infections. This case supports the view that N.fowleri increases its range during the year as temperatures rise during spring and summer months.[20]
In July 2014, a 9 year old Kansas girl, an avid water-skier, died from the disease after swimming in warm fresh water.[45][46]
Though no reports of illness had been made as of yet, in late August 2014, officials in St. John the Baptist Parish, Louisiana had to start flushing the water system because of Naegleria fowleri contamination, a process which will take two months, though the water has been deemed safe to drink. About 12,500 people are served by the water system, and while Naegleria fowleri is not pathogenic in the human digestive tract, parish officials are taking no chances.[47]
Venezuela
In 1998, in Venezuela, a sixteen-year-old male died a week after becoming infected by Naegleria fowleri.[48] There were two new cases reported in 2006. Two males, one ten years old and one twenty-three years old, both died within days of their hospital admittance.[49]
Research
Diagnostics
Current research is focused on development of real time PCR diagnostic methods. One method being developed involves monitoring the amplification process in real time with fluorescent-labeled hybridization probes targeting the MpC15 sequence – which is unique to N. fowleri.[50] Another group has multiplexed three real-time PCR reactions as a diagnostic for N. fowleri, as well as Acanthamoeba spp. and Balamuthia mandrillaris.[51] This could prove to be an efficient diagnostic test.
Pathogenicity factors
As no effective treatment for PAM has been found, the development of a therapeutic is an area of great research interest. Currently, much work is being done to determine what factor specific to N. fowleri makes it pathogenic and if these virulence factors can be targeted by drugs. One potential factor in motility of the "amoeba" is the protein coded by Nfa1. When the Nfa1 gene is expressed in non-pathogenic Naegleria gruberi and the amoebae are co-cultivated with target tissue cells, the protein is found to be located on the food cup which is responsible for ingestion of cells during feeding.[52] Following up that research, Nfa1 gene expression knockdown experiments were performed using RNA interference. In these experiments, double-stranded RNA targeting the Nfa1 sequence was introduced and subsequently expression levels of the gene product dramatically decreased.[53] This method could potentially be a technique applicable for knockdown of expression of pathogenicity factors in N. fowleri trophozoites.
Vaccine research
There is no vaccine to protect against N. fowleri infection and efforts are being made in vaccine research to find one. Current research shows intranasal administration of Cry1Ac protoxin alone or in combination with amoebic lysates increases protection against N. fowleri meningoencephalitis in mice. Studies are ongoing investigating whether the STAT6-induced Th2 immune response is essential for the resistance to N. fowleri infection, conferred by immunization with amoebic lysates plus Cry1Ac. Protected STAT6+/+-immunized mice elicited a Th2 type inclined immune response that produced predominantly humoral immunity; unprotected STAT6-/- mice exhibited a polarized Th1-type cellular response. These findings suggest that the STAT6-signalling pathway is critical for defense against N. fowleri infection. Immunization with Nfa1 protein on experimental murines PAM because of N. fowleri, BALB/c mice were intraperitoneally or intranasally immunized with a recombinant Nfa1 protein. The mean survival time of mice immunized intraperitoneally with rNfa1 protein was prolonged compared with controls (25.0 and 15.5 days, respectively).
See also
References
- ^ "The Centers for Disease Control and Prevention, Division of Parasitic Diseases – Naegleria fowleri - Primary Amoebic Meningoencephalitis (PAM) - General Information". Retrieved 2014-05-26.
- ^ "6 die from brain-eating amoeba after swimming". MSNBC. Associated Press. September 28, 2007.
- ^ Cetin N, Blackall D.Naegleria fowleri meningoencephalitis.Blood 2012 Apr 19;119(16):3658.PMID 22645743
- ^ a b c Marciano-Cabral, F (1988). "Biology of Naegleria spp". Microbiological reviews. 52 (1): 114–33. PMC 372708. PMID 3280964.
- ^ Chang, SL (1978). "Resistance of pathogenic Naegleria to some common physical and chemical agents". Applied and environmental microbiology. 35 (2): 368–75. PMC 242840. PMID 637538.
- ^ Fowler, M; Carter, RF (1965). "Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report". British Medical Journal. 2 (5464): 740–2. doi:10.1136/bmj.2.5464.734-a. PMC 1846173. PMID 5825411.
- ^ Butt, Cecil G. (1966). "Primary Amebic Meningoencephalitis". New England Journal of Medicine. 274 (26): 1473–6. doi:10.1056/NEJM196606302742605. PMID 5939846.
- ^ Symmers, WC (1969). "Primary amoebic meningoencephalitis in Britain". British Medical Journal. 4 (5681): 449–54. doi:10.1136/bmj.4.5681.449. PMC 1630535. PMID 5354833.
- ^ CDC – 01 This Page Has Moved: CDC Parasites Naegleria
- ^ Su MY, Lee MS,et al. A fatal case of Naegleria fowleri meningoencephalitis in Taiwan.Korean J Parasitol. 2013 Apr;51(2):203-6. doi: 10.3347/kjp.2013.51.2.203. Epub 2013 Apr 25. PMID 23710088
- ^ Gautam PL, Sharma S et al. A rare case of survival from primary amebic meningoencephalitis.Indian Crit Care med.2012 Jan;16(1):34-6. doi: 10.4103/0972-5229.94432.PMID 22557831
- ^ Marciano-Cabral, F; John, DT (1983). "Cytopathogenicity of Naegleria fowleri for rat neuroblastoma cell cultures: scanning electron microscopy study". Infection and immunity. 40 (3): 1214–7. PMC 348179. PMID 6852919.
- ^ Monsters Inside Me (2010-12-22). "Monsters Inside Me: The Brain-Eating Amoeba : Video : Animal Planet". Animal.discovery.com. Retrieved 2012-09-04.
- ^ a b c Centers for Disease Control and Prevention (CDC) (2008). "Primary amebic meningoencephalitis – Arizona, Florida, and Texas, 2007". MMWR. Morbidity and mortality weekly report. 57 (21): 573–7. PMID 18509301.
- ^ Bauman, Robert W. (2009). "Microbial Diseases of the Nervous System and Eyes". Microbiology, With Diseases by Body System (2nd ed.). San Francisco: Pearson Education. p. 617.
- ^ Reference 23
- ^ Kim, J.-H.; Jung, S.-Y.; Lee, Y.-J.; Song, K.-J.; Kwon, D.; Kim, K.; Park, S.; Im, K.-I.; Shin, H.-J. (2008). "Effect of Therapeutic Chemical Agents In Vitro and on Experimental Meningoencephalitis Due to Naegleria fowleri". Antimicrobial Agents and Chemotherapy. 52 (11): 4010–6. doi:10.1128/AAC.00197-08. PMC 2573150. PMID 18765686.
{{cite journal}}: Invalid|display-authors=9(help) - ^ Donald C. Lehman; Mahon, Connie; Manuselis, George (2006). Textbook of Diagnostic Microbiology (3rd ed.). Philadelphia: Saunders. ISBN 1-4160-2581-2.
{{cite book}}: CS1 maint: multiple names: authors list (link)[page needed] - ^ Pougnard, C.; Catala, P.; Drocourt, J.-L.; Legastelois, S.; Pernin, P.; Pringuez, E.; Lebaron, P. (2002). "Rapid Detection and Enumeration of Naegleria fowleri in Surface Waters by Solid-Phase Cytometry". Applied and Environmental Microbiology. 68 (6): 3102–7. doi:10.1128/AEM.68.6.3102-3107.2002. PMC 123984. PMID 12039772.
- ^ a b Kemble SK, Lynfield R, et al.Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism. Clin Infect Dis. 2012 Mar;54(6):805-9. doi: 10.1093/cid/cir961. Epub 2012 Jan 11.PMID 22238170
- ^ Kanwal, Farooqi M, Ali S, Ahmed SS.The paradox of primary amoebic meningoencephalitis--a rare disease, but commonly misdiagnosed.J PakMed Assoc2013 May;63(5):667.PMID 22238170
- ^ Reference 38
- ^ "Brain-eating amoeba victim: Sanford boy 'a ray of light'". Retrieved 2014-08-22.
- ^ Ccaronerva, L.; Novak, K. (1968). "Amoebic Meningoencephalitis: Sixteen Fatalities". Science. 160 (3823): 92–92. doi:10.1126/science.160.3823.92. PMID 5642317.
- ^ Shenoy S, Wilson G, Prashanth HV, et al.Primary meningoencephalitis by Naegleria fowleri: first reported case from Mangalore, South India. J Clin Microbiol. 2002 Jan;40(1):309-10.PMID 11773141
- ^ Hebbar S, Bairy I, et al.Fatal case of Naegleria fowleri meningo-encephalitis in an infant: case report.Ann Trop Paediatr. 2005 Sep;25(3):223-6.PMID 16156990
- ^ Gupta N, Bhaskar H, et al.Primary amoebic meningoencephalitis: first reported case from Rohtak, North India.National Institute of Communicable Diseases, New Delhi.PMID 20191204
- ^ Movahedi Z, Shokrollahi MR,et al. Primary amoebic meningoencephalitis in an Iranian infant.Case Rep Med. 2012;2012:782854. doi: 10.1155/2012/782854. Epub 2012 Jul 26.PMID 22899941
- ^ Cursons, R; Sleigh, J; Hood, D; Pullon, D (2003). "A case of primary amoebic meningoencephalitis: North Island, New Zealand". The New Zealand medical journal. 116 (1187): U712. PMID 14752540.
- ^ "Brain-eating amoeba: need for water chlorination stressed". Retrieved 2012-07-19.
- ^ "'Brain-eating amoeba' claims another life in Karachi accessdate = 2014-10-10".
{{cite web}}: Missing pipe in:|title=(help) - ^ cdc.gov – Primary Amebic Meningoencephalitis Caused by Naegleria fowleri, Karachi, Pakistan, 2011-07-08
- ^ Journal of Medical Microbiology. 02/2014; doi:10.1099/jmm.0.072306-0
- ^ CNS Neuroscience & Therapeutics /01/2014; doi:10.1111/cns.12225
- ^ Reference10
- ^ "The City of Bath, Somerset, UK". H2G2. Retrieved 2007-11-01.
- ^ Kilvington, Simon; John Beeching (June 1995). "Identification and epidemiological typing of Naegleria fowleri with DNA probes" (PDF). Applied and Environmental Microbiology. 61 (6): 2071–2078. PMC 167479. PMID 7793928. Retrieved 2007-10-31.
- ^ http://www.dailymail.co.uk/news/article-2689800/Kansas-girl-9-dies-rare-brain-eating-amoeba.html
- ^ "Naegleria – Frequently Asked Questions (FAQs)". Centers for Disease Control and Prevention. July 5, 2011. Retrieved 2011-08-17.
- ^ Joelving, Frederick (2012-08-28). "CDC strengthens brain-eating amoeba, neti pot link". Reuters. Retrieved 4 January 2013.
- ^ "CDC strengthens brain-eating ameba, neti pot link". Chicago Tribune. 2012-08-28.
- ^ http://www.nola.com/health/index.ssf/2013/09/st_bernard_water_system_tests.html
- ^ Gholipour, Bahar. "Brain-Eating Amoeba: How One Girl Survived". livescience. Retrieved 28 August 2013.
- ^ "Florida boy, 12, dies from brain-eating parasite, his family donated his organs wich were still good to save other lives". CNN. 2013-08-26. Retrieved 24 August 2013.
- ^ http://news.msn.com/us/girl-9-killed-by-brain-eating-parasite-in-water
- ^ http://www.cnn.com/2014/07/15/health/brain-eating-amoeba/index.html
- ^ http://news.msn.com/us/potentially-deadly-amoeba-found-in-louisiana-water
- ^ Rodríguez R, Méndez O, et al.Central nervous system infection by free-living amebas: report of 3 Venezuelan cases.Rev Neurol. 1998 Jun;26(154):1005-8.PMID 9658480
- ^ Petit F, Vilchez V, et al.Primary amebic meningoencephalitis: two new cases report from Venezuela. Arq Neuropsiquiatr 2006 Dec;64(4):1043-6.PMID 17221024
- ^ Maďarová, Lucia; Trnková, Katarína; Feiková, Soňa; Klement, Cyril; Obernauerová, Margita (2010). "A real-time PCR diagnostic method for detection of Naegleria fowleri". Experimental Parasitology. 126 (1): 37–41. doi:10.1016/j.exppara.2009.11.001. PMID 19919836.
- ^ Qvarnstrom, Y.; Visvesvara, G. S.; Sriram, R.; Da Silva, A. J. (2006). "Multiplex Real-Time PCR Assay for Simultaneous Detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri". Journal of Clinical Microbiology. 44 (10): 3589–95. doi:10.1128/JCM.00875-06. PMC 1594764. PMID 17021087.
- ^ Song, K; Jeong, S; Park, S; Kim, K; Kwon, M; Im, K; Pak, J; Shin, H (2006). "Naegleria fowleri: Functional expression of the Nfa1 protein in transfected Naegleria gruberi by promoter modification". Experimental Parasitology. 112 (2): 115–20. doi:10.1016/j.exppara.2005.10.004. PMID 16321386.
- ^ Jung, S; Kim, J; Lee, Y; Song, K; Kim, K; Park, S; Im, K; Shin, H (2008). "Naegleria fowleri: nfa1 gene knock-down by double-stranded RNAs". Experimental Parasitology. 118 (2): 208–13. doi:10.1016/j.exppara.2007.08.008. PMID 17904122.
External links
- Naegleria Infection Information Page from the Centers for Disease Control and Prevention
- Naegleria General Information from the website of the Centers for Disease Control and Prevention
- Naegleria from the The Tree of Life Web Project
