Alendronic acid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fosamax |
| Other names | alendronate sodium (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601011 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 0.6% |
| Metabolism | excreted unchanged |
| Elimination half-life | 126 months |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.128.415 |
| Chemical and physical data | |
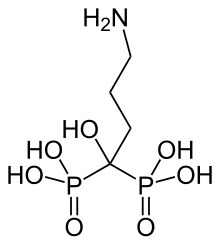
| Formula | C4H13NO7P2 |
| Molar mass | 249.097 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Alendronic acid (INN) or alendronate sodium, sold in the U.S. under the trade name Fosamax among others, is a bisphosphonate drug used for osteoporosis, osteogenesis imperfecta, and several other bone diseases. It is marketed alone as well as in combination with vitamin D.
Merck's U.S. patent on alendronate expired in 2008 and the drug is now available as a generic. It is the most widely prescribed bisphosphonate medicine in the United States.[citation needed]
Medical uses

- Prophylaxis and treatment of female osteoporosis
- Treatment of male osteoporosis
- Prevention and treatment of corticosteroid-associated osteoporosis together with supplements of calcium and vitamin D
- Paget's disease
- Treatment for Osteogenesis imperfecta in patients 18 years of age or older
- Dental implants coating (experimental)
Contraindications
Alendronate should not be used in:
- Acute inflammations of the gastrointestinal tract (esophagitis, gastritis, ulcerations)
- Clinically manifested osteomalacia
- Certain malformations and malfunctions of the esophagus (strictures, achalasia)
- Inability to stand, walk, or sit for 30 minutes after oral administration
- Renal impairment or chronic kidney disease as evidenced by a creatinine clearance below 30ml/min
- Hypersensitivity to alendronate or another ingredient in the product
- Hypocalcemia
- Pregnancy and breastfeeding
- Patients below 18 yrs. of age, as no clinical data exists for this population
Side effects
- Gastrointestinal tract:
- Ulceration and possible rupture of the esophagus; this may require hospitalization and intensive treatment. Gastric and duodenal ulceration may also occur.
- Esophageal cancer, a meta-analysis concluded that bisphosphonate treatment is not significantly associated with excess risk of esophageal cancer.[1][2]
- General: infrequent cases of skin rash, rarely manifesting as Stevens–Johnson syndrome and toxic epidermal necrolysis, eye problems (uveitis, scleritis) and generalized muscle, joint, and bone pain[3] (rarely severe) have been reported. In laboratory tests, decreased calcium and phosphate values may be seen but reflect expected action of the drug and are almost always not clinically relevant.
- Osteonecrosis of the jaw (deterioration of the temporomandibular joint or TMJ) may occur while on this drug, if dental work of any kind is carried out.[4] Although this side effect is uncommon, it occurs primarily in patients being administered intravenous bisphosphonates, with most cases being reported in cancer patients.[5][6]
- Bone: alendronate has been linked in long-term users to the development of low-impact femoral fractures.[7] Further, studies suggest that users of alendronate have an increase in the numbers of osteoclasts and develop giant, more multinucleated osteoclasts; the significance of this development is unclear.[8] Fosamax has been linked to a rare type of leg fracture that cuts straight across the upper thigh bone after little or no trauma (subtrochanteric fractures).[9]
Interactions
- Food and drugs containing large amounts of calcium, magnesium or aluminium (antacids) decrease the absorption of alendronate. At least half an hour should pass after intake of alendronate before eating dairy products or taking the supplement or drug.
- Highly active vitamin D analogues or fluorides: no data is available. Concomitant treatment should be avoided.
- The additional beneficial effect of HRT (hormone replacement therapy) with estrogens/progestins or raloxifene in postmenopausal women with osteoporosis remains to be elucidated, but no interactions have been seen. The combination is therefore possible, but controversial.
- Intravenous ranitidine increases the oral bioavailability of alendronate. No clinical consequences are known.
- The combination of NSAIDs and alendronate may increase the risk of gastric ulcers. Both these drugs have the potential to irritate the upper gastro-intestinal mucosa.
Pharmacology
Mechanism of action
Alendronate inhibits osteoclast-mediated bone-resorption. Like all bisphosphonates, it is chemically related to inorganic pyrophosphate, the endogenous regulator of bone turnover. But while pyrophosphate inhibits both osteoclastic bone resorption and the mineralization of the bone newly formed by osteoblasts, alendronate specifically inhibits bone resorption without any effect on mineralization at pharmacologically achievable doses. Its inhibition of bone-resorption is dose-dependent and approximately 1,000 times stronger than the equimolar effect of the first bisphosphonate drug, etidronate. Under therapy, normal bone tissue develops, and alendronate is deposited in the bone-matrix in a pharmacologically inactive form. For optimal action, enough calcium and vitamin D are needed in the body in order to promote normal bone development. Hypocalcemia should, therefore, be corrected before starting therapy.
Etidronate has the same disadvantage as pyrophosphate in inhibiting mineralization, but all of the potent N-containing bisphosphonates including Alendronate and also risedronate, ibandronate, and zoledronate, do not.
Pharmacokinetics
As with all potent bisphosphonates, the fraction of the drug that reaches the circulatory system intact (systemic bioavailability) after oral dosing is low, averaging only 0.6–0.7% in women and in men under fasting conditions. Intake together with meals and beverages other than water further reduces the bioavailability. The absorbed drug rapidly partitions, with approximately 50% binding to the exposed bone surface; the remainder is excreted unchanged by the kidneys. Unlike with most drugs, the strong negative charge on the two phosphonate moieties limits oral bioavailability, and, in turn, the exposure to tissues other than bone is very low. After absorption in the bone, alendronate has an estimated terminal elimination half-life of 10 years.[10]
| Bisphosphonate | Relative potency |
|---|---|
| Etidronate | 1 |
| Tiludronate | 10 |
| Pamidronate | 100 |
| Aledronate | 100-500 |
| Ibandronate | 500-1000 |
| Risedronate | 1000 |
| Zoledronate | 5000 |
References
- ^ Sun K, Liu JM, Sun HX, Lu N, Ning G (October 2012). "Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies". Osteoporosis International. 24 (1): 279–86. doi:10.1007/s00198-012-2158-8. PMID 23052941.
- ^ Haber SL, McNatty D (March 2012). "An evaluation of the use of oral bisphosphonates and risk of esophageal cancer". Ann Pharmacother. 46 (3): 419–23. doi:10.1345/aph.1Q482. PMID 22333262.
- ^ FDA Patient Safety News, March 2008
- ^ Fosamax product description, Merck & Co
- ^ Pazianas, M.; Miller P; Blumentals WA; Bernal M; Kothawala P (August 29, 2007). "A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics". Clinical Therapy. 29 (8): 1548–58. doi:10.1016/j.clinthera.2007.08.008. PMID 17919538.
- ^ Carini, F.; Barbano L; Saggese V; Monai D; Porcaro G. (2012-04-03). "Multiple systemic diseases complicated by bisphosphonate osteonecrosis: a case report". Ann Stomatol (Roma). 3 (2 Suppl): 32–6. PMC 3512552. PMID 23285320.
- ^ Lenart BA, Lorich DG, Lane JM (March 2008). "Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate". N. Engl. J. Med. 358 (12): 1304–6. doi:10.1056/NEJMc0707493. PMID 18354114.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Weinstein RS, Roberson PK, Manolagas SC (January 2009). "Giant osteoclast formation and long-term oral bisphosphonate therapy". N. Engl. J. Med. 360 (1): 53–62. doi:10.1056/NEJMoa0802633. PMC 2866022. PMID 19118304.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Kwek EB, Goh SK, Koh JS, Png MA, Howe TS (February 2008). "An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy?". Injury. 39 (2): 224–31. doi:10.1016/j.injury.2007.08.036. PMID 18222447.
- ^ Shinkai I, Ohta Y (January 1996). "New drugs--reports of new drugs recently approved by the FDA. Alendronate". Bioorg. Med. Chem. 4 (1): 3–4. doi:10.1016/0968-0896(96)00042-9. PMID 8689235.
- ^ D.,, Tripathi, K. Essentials of medical pharmacology (Seventh edition ed.). New Delhi. ISBN 9789350259375. OCLC 868299888.
{{cite book}}:|edition=has extra text (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link)
