Colorectal cancer
| Colorectal cancer | |
|---|---|
| Specialty | Oncology |
Colorectal cancer, also called colon cancer or bowel cancer, includes cancerous growths in the colon, rectum and appendix. It is the third most common form of cancer and the second leading cause of death among cancers in the Western world. Many colorectal cancers are thought to arise from adenomatous polyps in the colon. These mushroom-like growths are usually benign, but some may develop into cancer over time. The majority of the time, the diagnosis of localized colon cancer is through colonoscopy. Therapy is usually through surgery, which in many cases is followed by chemotherapy.
Symptoms
Frequently, the patient may be asymptomatic. This is one reason why many organizations recommend periodic screening for the disease with fecal occult blood testing and colonoscopy. When symptoms do occur, they depend on the site of the lesion. Generally speaking, the nearer the lesion is to the anus, the more bowel symptoms there will be, such as:
- Change in bowel habits
- change in frequency [(constipation and/or (spurious) diarrhoea),
- change in the quality of stools
- change in consistency of stools
- bloody stools or rectal bleeding
- Stools with mucus
- Tarry stools (melena)
- Feeling of incomplete defecation (Tenesmus) (only associated with rectal cancer)
- Reduction in calibre of faeces (only associated with rectal cancer)
- Bowel obstruction (rare)
Constitutional Symptoms
Especially in the cases of cancer in the ascending colon, sometimes only the less specific constitutional symptoms will be found:
- Anemia, with symptoms such as dizziness, malaise and palpitations. Clinically there will be pallor and a complete blood picture will confirm the low hemoglobin level.
- Anorexia
- Asthenia, weakness
- Unexplained weight loss.
Metastatic Symptoms
There may also be symptoms attributed to distant metastasis:
- Shortness of breath as in lung metastasis
- Epigastric or right upper quadrant pain, as in liver metastasis. Rarely can there be jaundice if the secondary lesion compromises the bile outflow. Clinically there might be hepatomegaly.
Risk factors
The lifetime risk of developing colon cancer in the United States is about 7%. Certain factors increase a person's risk of developing the disease. These include:
- Age. The risk of developing colorectal cancer increases with age. Most cases occur in the 60s and 70s, while cases before age 50 are uncommon unless a family history of early colon cancer is present.
- Polyps of the colon, particularly adenomatous polyps, are a risk factor for colon cancer. The removal of colon polyps at the time of colonoscopy reduces the subsequent risk of colon cancer.
- History of cancer. Individuals who have previously been diagnosed and treated for colon cancer are at risk for developing colon cancer in the future. Women who have had cancer of the ovary, uterus, or breast are at higher risk of developing colorectal cancer.
- Heredity:
- Family history of colon cancer, especially in a close relative before the age of 55 or multiple relatives
- Familial adenomatous polyposis (FAP) carries a near 100% risk of developing colorectal cancer by the age of 40 if untreated
- Hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome
- Long-standing ulcerative colitis or Crohn's disease of the colon, approximately 30% after 25 years if the entire colon is involved
- Smoking. Smokers are more likely to die of colorectal cancer than non-smokers. An ACS study found that "Women who smoked were more than 40% more likely to die from colorectal cancer than women who never had smoked. Male smokers had more than a 30% increase in risk of dying from the disease compared to men who never had smoked."[1]
- Diet. Studies show that a diet high in red meat[1] and low in fresh fruit, vegetables, poultry and fish increases the risk of colorectal cancer. In June 2005, a study by the European Prospective Investigation into Cancer and Nutrition suggested that diets high in red and processed meat, as well as those low in fiber, are associated with an increased risk of colorectal cancer. Individuals who frequently ate fish showed a decreased risk.[2] However, other studies have cast doubt on the claim that diets high in fiber decrease the risk of colorectal cancer.[3] The nature of the relationship between dietary fiber and risk of colorectal cancer remains controversial.
- Physical inactivity. People who are physically active are at lower risk of developing colorectal cancer.
- Virus. Exposure to some viruses (such as particular strains of human papilloma virus) may be associated with colorectal cancer.
- Alcohol. "Heavy alcohol use may also increase the risk of colorectal cancer" [2]
- Primary sclerosing cholangitis offers a risk independent to ulcerative colitis
- Low selenium.
Alcohol
This November 2006 may be in need of reorganization to comply with Wikipedia's layout guidelines. |
The National Cancer Institute does not list alcohol as a risk factor.[3]
The NIAAA reports that, "Epidemiologic studies have found a small but consistent dose-dependent association between alcohol consumption and colorectal cancer[4][5]even when controlling for fiber and other dietary factors.[6][7] Despite the large number of studies, however, causality cannot be determined from the available data."[8]
"Heavy alcohol use may also increase the risk of colorectal cancer" (NCI). One study found that "People who drink more 30 grams of alcohol per day (and especially those who drink more than 45 grams per day) appear to have a slightly higher risk for colorectal cancer."[9][10] Another found that "The consumption of one or more alcoholic beverages a day at baseline was associated with approximately a 70% greater risk of colon cancer."[11][12][13]
One study found that "While there was a more than twofold increased risk of significant colorectal neoplasia in people who drink spirits and beer, people who drank wine had a lower risk. In our sample, people who drank more than eight servings of beer or spirits per week had at least a one in five chance of having significant colorectal neoplasia detected by screening colonoscopy.".[14]
Other research suggests that "to minimize your risk of developing colorectal cancer, it's best to drink in moderation"[8]
Drinking may be a cause of earlier onset of colorectal cancer.[15]
Diagnosis, screening and monitoring
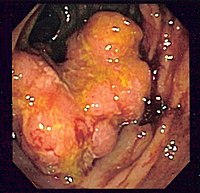
Colorectal cancer can take many years to develop and early detection of colorectal cancer greatly improves the chances of a cure. Therefore, screening for the disease is recommended in individuals who are at increased risk. There are several different tests available for this purpose.
- Digital rectal exam (DRE): The doctor inserts a lubricated, gloved finger into the rectum to feel for abnormal areas. It only detects tumors large enough to be felt in the distal part of the rectum and is not really a screening test.
- Fecal occult blood test (FOBT): a test for blood in the stool.
- Endoscopy:
- Sigmoidoscopy: A lighted probe (sigmoidoscope) is inserted into the rectum and lower colon to check for polyps and other abnormalities.
- Colonoscopy: A lighted probe called a colonoscope is inserted into the rectum and the entire colon to look for polyps and other abnormalities that may be caused by cancer. A colonoscopy has the advantage that if polyps are found during the procedure they can be immediately removed. Tissue can also be taken for biopsy.
- Double contrast barium enema (DCBE): First, an overnight preparation is taken to cleanse the colon. An enema containing barium sulfate is administered, then air is insufflated into the colon, distending it. The result is a thin layer of barium over the inner lining of the colon which is visible on X-ray films. A cancer or a precancerous polyp can be detected this way. This technique can miss the (less common) flat polyp.
- Virtual colonoscopy replaces X-ray films in the double contrast barium enema (above) with a special computed tomography scan and requires special workstation software in order for the radiologist to interpret. This technique is approaching colonoscopy in sensitivity for polyps. However, any polyps found must still be removed by standard colonoscopy.
- Standard computed axial tomography is an x-ray method that can be used to determine the degree of spread of cancer, but is not sensitive enough to use for screening. Some cancers are found in CAT scans performed for other reasons.
- Blood tests: Measurement of the patient's blood for elevated levels of certain proteins can give an indication of tumor load. In particular, high levels of carcinoembryonic antigen CEA in the blood can indicate metastasis of adenocarcinoma. These tests are frequently false positive or false negative, and are not recommended for screening.
- Genetic counseling and genetic testing for families who may have a hereditary form of colon cancer, such as hereditary nonpolyposis colorectal cancer (HNPCC) or familial adenomatous polyposis (FAP).
- Positron emission tomography (PET) is a 3-dimensional scanning technology where a radioactive sugar is injected into the patient, the sugar collects in tissues with high metabolic activity, and an image is formed by measuring the emission of radiation from the sugar. Because cancer cells often have very high metabolic rate, this can be used to differentiate benign and malignant tumors. PET is not used for screening and does not (yet) have a place in routine workup of colorectal cancer cases.
- Stool DNA Testing is an emerging technology in screening for colorectal cancer. Pre-malignant adenomas and cancers shed DNA markers from their cells which are not degraded during the digestive process and remain stable in the stool. Capture, followed by Polymerase Chain Reaction amplifies the DNA to detectable levels for assay. Clinical studies have shown a cancer detection sensitivity of 71%-91%.[16]
Pathology

The pathology of the tumor is usually reported from the analysis of tissue taken from a biopsy or surgery. A pathology report will usually contain a description of cell type and grade. The most common colon cancer cell type is adenocarcinoma which accounts for 95% of cases. Other, rarer types include lymphoma and squamous cell carcinoma.
Cancers on the right side (ascending colon and cecum) tend to be exophytic, that is, the tumour grows outwards from one location in the bowel wall. This very rarely causes obstruction of feces, and presents with symptoms such as anemia. Left-sided tumours tend to be circumferential, and can obstruct the bowel much like a napkin ring.
Histopathology: Adenocarcinoma is a malignant epithelial tumor, originating from glandular epithelium of the colorectal mucosa. It invades the wall, infiltrating the muscularis mucosae, the submucosa and thence the muscularis propria. Tumor cells describe irregular tubular structures, harboring pluristratification, multiple lumens, reduced stroma ("back to back" aspect). Sometimes, tumor cells are discohesive and secrete mucus, which invades the interstitium producing large pools of mucus/colloid (optically "empty" spaces) - mucinous (colloid) adenocarcinoma, poorly differentiated. If the mucus remains inside the tumor cell, it pushes the nucleus at the periphery - "signet-ring cell." Depending on glandular architecture, cellular pleomorphism, and mucosecretion of the predominant pattern, adenocarcinoma may present three degrees of differentiation: well, moderately, and poorly differentiated. 1
Staging
Definitive staging can only be done after surgery has been performed and the pathology report reviewed. Exception to this includes: Colonoscopic polypectomy of a malignant pedunculated polyp with minimal invasion. Preoperative staging of rectal cancers may be done with endoscopic ultrasound. Adjuncts to staging of metastasis include: Abdominal Ultrasound, CT, PET Scanning along with other imaging studies.
TNM or Dukes'
Colon cancer staging is an estimate of the condition of a particular cancer for diagnostic and research purposes. The systems for staging colorectal cancers largely depend on the extent of local invasion, the degree of lymph node involvement and whether there is distant metastasis.
The most common currently used system for staging is the TNM system, though many doctors still use the older Dukes system. The TNM system assigns a number:
- T - The degree of invasion of the intestinal wall
- T0 - no evidence of tumor
- Tis- cancer in situ (tumor present, but no invasion)
- T1 - invasion through submucosa into lamina propria (basement membrane invaded)
- T2 - invasion into the muscularis propria
- T3 - invasion through the muscularis propria OR to adjacent mucosa
- T4 - invasion completely through the wall of the colon
- N - the degree of lymphatic node involvement
- N0 - no lymph nodes involved
- N1 - one to three nodes involved
- N2 - four or more nodes involved
- M - the degree of metastasis
- M0 - no metastasis
- M1 - metastasis present
Dukes' classification, first proposed by Dr Cuthbert E. Dukes in 1932, identifies the stages as:[17]
- A - Tumour confined to the intestinal wall
- B - Tumour invading into intestinal wall
- C - With lymph node(s) involvement
- D - With distant metastasis
AJCC stage groupings
The stage of a cancer is usually quoted as a number I,II,III,IV derived from the TNM value grouped by prognosis; a higher number indicates a more advanced cancer and a likely worse outcome.
- Stage 0
- Tis, N0, M0
- Stage I
- T1, N0, M0
- T2, N0, M0
- Stage IIA
- T3, N0, M0
- Stage IIB
- T4, N0, M0
- Stage IIIA
- T1, N1, M0
- T2, N1, M0
- Stage IIIB
- T3, N1, M0
- T4, N1, M0
- Stage IIIC
- Any T, N2, M0
- Stage IV
- Any T, Any N, M1
Pathogenesis
Colorectal cancer is a disease originating from the epithelial cells lining the gastrointestinal tract. Mutations in specific DNA sequences, among which are included the APC, K-Ras and p53 genes, lead to unrestricted cell division. Various causes for these mutations are inborn genetic aberrations, tobacco smoking, environmental, and possibly viral causes. The exact reason why a diet high in fiber might prevent colorectal cancer remains uncertain. Chronic inflammation, as in inflammatory bowel disease, may predispose patients to malignancy.
Treatment
The treatment depends on the staging of the cancer. When colorectal cancer is caught at early stages (with little spread) it can be curable. However when it is detected at later stages (when distant metastases are present) it is less likely to be curable.
Surgery remains the primary treatment while chemotherapy and/or radiotherapy may be recommended depending on the individual patient's staging and other medical factors.
Surgery
Surgeries can be categorised into curative, palliative, bypass, fecal diversion, or open-and-close.
Curative Surgical treatment can be offered if the tumor is localized.
- Very early cancer that develops within a polyp can often be cured by removing the polyp (i.e., polypectomy) at the time of colonoscopy.
- In colon cancer, a more advanced tumor typically requires surgical removal of the section of colon containing the tumor with sufficient margins, and radical en-bloc resection of mesentery and lymph nodes to reduce local recurrence (i.e., colectomy). If possible, the remaining parts of colon are anastomosed together to create a functioning colon. In cases when anastomosis is not possible, a stoma (artificial orifice) is created.
- Curative surgery on rectal cancer includes total mesorectal excision (anterior resection) or abdominoperineal excision.
In case of multiple mestatasis, palliative resection of the primary tumour is still offered in order to reduce further morbidity caused by tumor bleeding, invasion, and its catabolic effect. Surgical removal of isolated liver metastases is, however, common; improved chemotherapy has increased the number of patients who are offered surgical removal of isolated liver metastases.
If the tumor invaded into adjacent vital structures which makes excision technically difficult, the surgeons may prefer to bypass the tumor (ileotransverse bypass) or to do a proximal fecal diversion through a stoma.
The worst case would be an open-and-close surgery, when surgeons find the tumor unresectable and the small bowel involved; any more procedures would do more harm than good to the patient.
Laparoscopic-assisted colectomy is a minimally-invasive technique that can reduce the size of the incision, minimize the risk of infection, and reduce post-operative pain.
As with any surgical procedure, colorectal surgery may result in complications including
- wound infection
- anastomosis breakdown, leading to abscess or fistula formation, and/or peritonitis
- bleeding with or without hematoma formation
- adhesions resulting in bowel obstruction (especially small bowel)
- blind loop syndrome as in bypass surgery.
- adjacent organ injury; most commonly to the small intestine, ureters, spleen, or bladder
Chemotherapy
Chemotherapy is used to reduce the likelihood of metastasis developing, shrink tumor size, or slow tumor growth. Chemotherapy is often applied after surgery (adjuvant), before surgery (neo-adjuvant), or as the primary therapy if surgery is not indicated (palliative). The treatments listed here have been shown in clinical trials to improve survival and/or reduce mortality rate and have been approved for use by the US Food and Drug Administration.
- Adjuvant (after surgery) chemotherapy. One regimen involves the combination of infusional 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX)
- 5-fluorouracil (5-FU) or Capecitabine (Xeloda®)
- Leucovorin (LV, Folinic Acid)
- Oxaliplatin (Eloxatin®)
- Chemotherapy for metastatic disease. Commonly used first line chemotherapy regimens involve the combination of infusional 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) with bevacizumab or infusional 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI) with bevacizumab
- 5-fluorouracil (5-FU) or Capecitabine
- Leucovorin (LV, Folinic Acid)
- Irinotecan (Camptosar®)
- Oxaliplatin (Eloxatin®)
- Bevacizumab (Avastin®)
- Cetuximab (Erbitux®)
- In clinical trials for treated/untreated metastatic disease. [4]
- Bortezomib (Velcade®)
- Panitumumab (Vectibix)
- Oblimersen (Genasense®, G3139)
- Gefitinib and Erlotinib (Tarceva®)
- Topotecan (Hycamtin®)
Radiation therapy
Radiotherapy is not used routinely in colorectal cancer, as it could lead to radiation enteritis, and is difficult to target specific portions of the colon. Indications included:
- Colon cancer
- pain relief and palliation - targeted at metastatic tumor deposits if they compress vital structures and/or cause pain.
- Rectal cancer
- neoadjuvant - downgrade the tumor to increase resectability
- adjuvant - where a tumor perforates the colon as judged by the surgeon or the pathologist (Dukes C tumour), guided by surgical clips
- palliative - kill tumor tissue when surgery is not indicated
Sometimes chemotherapy agents are used to increase the effectiveness of radiation by sensitizing tumor cells if present.
Immunotherapy
Bacillus Calmette-Guérin (BCG) is being investigated as a adjuvant mixed with autologous tumor cells in immunotherapy for colorectal cancer.[18]
Support therapies
Cancer diagnosis very often results in an enormous change in the patient's psychological wellbeing. Various support resources are available from hospitals and other agencies which provide counseling, social service support, cancer support groups, and other services. These services help to mitigate some of the difficulties of integrating a patient's medical complications into other parts of their life.
Prognosis
Survival is directly related to detection and the type of cancer involved. Survival rates for early stage detection is about 5 times that of late stage cancers. CEA level is also directly related to the prognosis of disease, since its level correlates with the bulk of tumor tissue.
Follow-up
Follow-up aims at diagnosing metachronise lesion(s) or distant metastasis in the early stage. History taking and physical examination every 3 to 6 months for three years after surgery. CEA every 2 to 3 months for two or more years in patients who have had resection of liver metastasis. Colonoscopy looking for synchronise lesion(s) should be done shortly after surgery if preoperatively the scope cannot pass through the tumor; otherwise it should be done every 3 to 5 years. ASCO recommends against other routine follow-up tests such as Chest X-Ray, Ultrasound, CT, etc.
Prevention
Most colorectal cancers should be preventable, through increased surveillance, improved lifestyle, and, probably, the use of dietary chemopreventive agents.
Surveillance
Most colorectal cancer arise from adenomatous polyps. These lesions can be detected and removed during colonoscopy. Studies show this procedure would decrease by > 80% the risk of cancer death, provided it is started by the age of 50, and repeated every 5 or 10 years.[19]
As per current guidelines under National Comprehensive Cancer Network at [www.NCCN.org], in average risk individuals with negative family history of colon cancer and personal history negative for adenomas or Inflammatory Bowel diseases, flexible sigmoidoscopy every 5 years with fecal occult blood testing annually or double contrast barium enema are other options acceptable for screening rather than colonoscopy every 10 years (which is currently the Gold-Standard of care).
Lifestyle
The comparison of colorectal cancer incidence in various countries strongly suggests that sedentarity, overeating (i.e., high caloric intake), and perhaps a diet high in meat (red or processed) could increase the risk of colorectal cancer. In contrast, physical exercise, and eating plenty of fruits and vegetables would decrease cancer risk, probably because they contain protective phytochemicals. Eating whole apples, including the skin, offers some anticancer benefits.[20] Accordingly, lifestyle changes could decrease the risk of colorectal cancer as much as 60-80%.[21]
Chemoprevention
More than 200 agents, including the above cited phytochemicals, and other food components like calcium or folic acid (a B vitamin), and NSAIDs like aspirin, are able to decrease carcinogenesis in preclinical models: Some studies show full inhibition of carcinogen-induced tumours in the colon of rats. Other studies show strong inhibition of spontaneous intestinal polyps in mutated mice (Min mice). Chemoprevention clinical trials in human volunteers have shown smaller prevention, but few intervention studies have been completed today. Calcium and aspirin supplements, given for 3 to 5 years after the removal of a polyp, modestly decreased the recurrence of polyps in volunteers (by 15-20%). The "chemoprevention database"[5] shows the results of all published scientific studies of chemopreventive agents, in people and in animals.
Mathematical modeling
Colorectal cancer has been for years subject of mathematical modeling.[22] For a comprehensive overview of current computational approaches on colorectal cancer see the Integrative Biology web page.
References
- ^ Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, Rodriguez C, Sinha R, Calle EE. Meat consumption and risk of colorectal cancer. JAMA 2005;293:172-82. PMID 15644544.
- ^ National Cancer Institute (NCI) Cancer Trends Progress Report Alcohol Consumption
- ^ Colorectal Cancer: Who's at Risk?
- ^ Longnecker, M.P. Alcohol consumption in relation to risk of cancers of the breast and large bowel. Alcohol Health & Research World 16(3)':223-229, 1992.
- ^ Longnecker, M.P.; Orza, M.J.; Adams, M.E.; Vioque, J.; and Chalmers, T.C. A meta-analysis of alcoholic beverage consumption in relation to risk of colorectal cancer Cancer Causes and Control 1(1):59-68, 1990.
- ^ Kune, S.; Kune, G.A.; and Watson, L.F. Case-control study of alcoholic beverages as etiological factors: The Melbourne Colorectal Cancer Study Nutrition and Cancer 9(1):43-56, 1987.
- ^ Potter, J.D., and McMichael, A.J. Diet and cancer of the colon and rectum: A case-control study Journal of the National Cancer Institute 76(4):557-569, 1986.
- ^ a b National Institute on Alcohol Abuse and Alcoholism Alcohol and Cancer - Alcohol Alert No. 21-1993
- ^ Alcohol Consumption and the Risk for Colorectal Cancer
- ^ Alcohol Intake and Colorectal Cancer: A Pooled Analysis of 8 Cohort Studies
- ^ Boston University "Alcohol May Increase the Risk of Colon Cancer"
- ^ Su LJ, Arab L. Alcohol consumption and risk of colon cancer: evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-Up Study. Nutr and Cancer. 2004;50(2):111–119.
- ^ Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Holmberg L, Kim DH, Malila N, Miller AB, Pietinen P, Rohan TE, Sellers TA, Speizer FE, Willett WC, Wolk A, Hunter DJ Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies Ann Intern Med 2004 Apr 20;140(8):603-13
- ^ Joseph C. Anderson, Zvi Alpern, Gurvinder Sethi, Catherine R. Messina, Carole Martin, Patricia M. Hubbard, Roger Grimson, Peter F. Ells, and Robert D. Shaw Prevalence and Risk of Colorectal Neoplasia in Consumers of Alcohol in a Screening Population Am J Gastroenterol Volume 100 Issue 9 Page 2049 Date September 2005
- ^ Brown, Anthony J. Alcohol, tobacco, and male gender up risk of earlier onset colorectal cancer
- ^ B. Greenwald (2006). "The DNA Stool Test - An Emerging Technology in Colorectal Cancer Screening".
{{cite web}}: Check|url=value (help) - ^ Dukes CE. The classification of cancer of the rectum. Journal of Pathological Bacteriology 1932;35:323.
- ^ Mosolits S, Nilsson B, Mellstedt H. Towards therapeutic vaccines for colorectal carcinoma: a review of clinical trials., Expert Rev. Vaccines, 2005;4:329-50. PMID 16026248.
- ^ Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, Ackroyd F, Shike M, Kurtz RC, Hornsby-Lewis L, Gerdes H, Stewart ET, The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329:1977-81. PMID 8247072.
- ^ Liu RH et al, Phytochemicals in apples are found to provide anticancer and anti-oxidant benefits. Link.
- ^ Cummings JH, Bingham SA. Diet and the prevention of cancer. BMJ 1998;317:1636-40. Fulltext. PMID 9848907.
- ^ van Leeuwen I, Byrne H, Jensen O, King J (2006). "Crypt dynamics and colorectal cancer: advances in mathematical modelling". Cell Prolif. 39 (3): 157–81. PMID 16671995.
{{cite journal}}: CS1 maint: multiple names: authors list (link)Full text
See also
External links
- National Cancer Institute (Cancer.gov) colorectal cancer
- Current clinical trials
- Complementary medical clinical trials
- Photos at: Atlas of Pathology
- Bowel Cancer UK (charity)
- Tackle Colon Cancer
- National Comprehensive Cancer Network (Colon Cancer Screening)
- National Comprehensive Cancer Network (Colon Cancer)
- People Living With Cancer (PLWC): Colorectal Cancer
- Beating Bowel Cancer (UK charity)
