Cervical cancer: Difference between revisions
m Reverted 1 edit by 72.70.42.186 (talk) to last revision by 112.79.47.48. (TW) |
→Screening: c/e |
||
| (One intermediate revision by the same user not shown) | |||
| Line 88: | Line 88: | ||
==Prevention {{Anchor | prevention}}== |
==Prevention {{Anchor | prevention}}== |
||
===Screening=== |
===Screening=== |
||
The widespread introduction of [[cervical screening]] by the [[pap test|Papanicolaou test]], or ''Pap smear'' for cervical cancer has been credited with dramatically reducing the incidence and mortality of cervical cancer in developed countries.<ref name="pmid10735343">{{cite journal | author = Canavan TP, Doshi NR | title = Cervical cancer | journal = Am Fam Physician | volume = 61 | issue = 5 | pages = 1369–76 | year = 2000 | pmid = 10735343 | doi = | url = http://www.aafp.org/afp/20000301/1369.html }}</ref> Pap smear screening every 3–5 years with appropriate follow-up can reduce cervical cancer incidence by up to 80%.<ref name="Arbyn10" /> Abnormal results may suggest the presence of [[cervical intraepithelial neoplasia|pre cancerous changes]] allowing examination and possible preventive treatment. If precancerous disease or cervical cancer is detected early, it can be monitored or treated relatively noninvasively, with little impairment of fertility. |
The widespread introduction of [[cervical screening]] by the [[pap test|Papanicolaou test]], or ''Pap smear'' for cervical cancer has been credited with dramatically reducing the incidence and mortality of cervical cancer in developed countries.<ref name="pmid10735343">{{cite journal | author = Canavan TP, Doshi NR | title = Cervical cancer | journal = Am Fam Physician | volume = 61 | issue = 5 | pages = 1369–76 | year = 2000 | pmid = 10735343 | doi = | url = http://www.aafp.org/afp/20000301/1369.html }}</ref> Pap smear screening every 3–5 years with appropriate follow-up can reduce cervical cancer incidence by up to 80%.<ref name="Arbyn10" /> Abnormal results may suggest the presence of [[cervical intraepithelial neoplasia|pre cancerous changes]] allowing examination and possible preventive treatment. If precancerous disease or cervical cancer is detected early, it can be monitored or treated relatively noninvasively, with little impairment of fertility.{{cn}} Personal invitations encouraging women to get screened are effective at increasing the likelihood they will do so. Educational materials also help increase the likelihood women will go for screening, but they are not as effective as invitations.<ref>{{Cite journal | last1 = Everett | first1 = T. | last2 = Bryant | first2 = A. | last3 = Griffin | first3 = MF. | last4 = Martin-Hirsch | first4 = PP. | last5 = Forbes | first5 = CA. | last6 = Jepson | first6 = RG. | title = Interventions targeted at women to encourage the uptake of cervical screening. | journal = Cochrane Database Syst Rev | volume = | issue = 5 | pages = CD002834 | month = | year = 2011 | doi = 10.1002/14651858.CD002834.pub2 | PMID = 21563135 }}</ref> |
||
Cervical cancer screening is typically recommended starting at age 21.<ref>{{cite journal | author = Saslow D, Solomon, D, Lawson, HW, Killackey, M, Kulasingam, SL, Cain, J, Garcia, FA, Moriarty, AT, Waxman, AG, Wilbur, DC, Wentzensen, N, Downs LS, Jr, Spitzer, M, Moscicki, AB, Franco, EL, Stoler, MH, Schiffman, M, Castle, PE, Myers, ER | title = American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer | journal = CA: a cancer journal for clinicians | volume = 62 | issue = 3 | pages = 147–72 | year = 2012 | pmid = 22422631 | doi = 10.3322/caac.21139 }}</ref><ref>{{cite journal | author = Moyer VA, on behalf of the U.S. Preventive Services Task, Force | title = Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement | journal = Annals of internal medicine | volume = | issue = | pages = | year = 2012 | pmid = 22422943 | doi = 10.1059/0003-4819-156-12-201206190-00424 }}</ref> Recommendations for how often a Pap smear should be done vary from once a year to once every five years, in the absence of abnormal results.<ref name=Arbyn10>{{cite journal | author = Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L | title = European Guidelines for Quality Assurance in Cervical Cancer Screening. Second Edition—Summary Document | journal = Annals of Oncology | volume = 21 | issue = 3 | pages = 448–458 | year = 2010 | pmid = 20176693 | pmc = 2826099 | doi = 10.1093/annonc/mdp471 }}</ref> Guidelines vary on how long to continue screening, but well screened women who have not had abnormal smears can stop screening about age 60 to 70.<ref name="USPSTF">{{cite web| author = U.S. Preventive Services Task Force | year = 2003 | url =http://www.ahrq.gov/clinic/3rduspstf/cervcan/cervcanrr.htm | title = Screening for Cervical Cancer: Recommendations and Rationale. AHRQ Publication No. 03-515A | publisher = Agency for Healthcare Research and Quality | location=Rockville, MD. | accessdate = June 5, 2010.}}</ref><ref name=ACS>{{cite journal | author = Saslow D, Runowicz CD, Solomon D, ''et al'' | title = American Cancer Society guideline for the early detection of cervical neoplasia and cancer | journal = CA: a cancer journal for clinicians | volume = 52 | issue = 6 | pages = 342–62 | year = 2002 | pmid = 12469763 | doi = 10.3322/canjclin.52.6.342 }}</ref><ref>{{cite journal | author = Strander B | title = At what age should cervical screening stop? | journal = Brit Med J | volume = 338 | issue = | pages = b809| year = 2009 | pmid = 19395422| doi = 10.1136/bmj.b809 }}</ref> |
Cervical cancer screening is typically recommended starting at age 21.<ref>{{cite journal | author = Saslow D, Solomon, D, Lawson, HW, Killackey, M, Kulasingam, SL, Cain, J, Garcia, FA, Moriarty, AT, Waxman, AG, Wilbur, DC, Wentzensen, N, Downs LS, Jr, Spitzer, M, Moscicki, AB, Franco, EL, Stoler, MH, Schiffman, M, Castle, PE, Myers, ER | title = American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer | journal = CA: a cancer journal for clinicians | volume = 62 | issue = 3 | pages = 147–72 | year = 2012 | pmid = 22422631 | doi = 10.3322/caac.21139 }}</ref><ref>{{cite journal | author = Moyer VA, on behalf of the U.S. Preventive Services Task, Force | title = Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement | journal = Annals of internal medicine | volume = | issue = | pages = | year = 2012 | pmid = 22422943 | doi = 10.1059/0003-4819-156-12-201206190-00424 }}</ref> Recommendations for how often a Pap smear should be done vary from once a year to once every five years, in the absence of abnormal results.<ref name=Arbyn10>{{cite journal | author = Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L | title = European Guidelines for Quality Assurance in Cervical Cancer Screening. Second Edition—Summary Document | journal = Annals of Oncology | volume = 21 | issue = 3 | pages = 448–458 | year = 2010 | pmid = 20176693 | pmc = 2826099 | doi = 10.1093/annonc/mdp471 }}</ref> Guidelines vary on how long to continue screening, but well screened women who have not had abnormal smears can stop screening about age 60 to 70.<ref name="USPSTF">{{cite web| author = U.S. Preventive Services Task Force | year = 2003 | url =http://www.ahrq.gov/clinic/3rduspstf/cervcan/cervcanrr.htm | title = Screening for Cervical Cancer: Recommendations and Rationale. AHRQ Publication No. 03-515A | publisher = Agency for Healthcare Research and Quality | location=Rockville, MD. | accessdate = June 5, 2010.}}</ref><ref name=ACS>{{cite journal | author = Saslow D, Runowicz CD, Solomon D, ''et al'' | title = American Cancer Society guideline for the early detection of cervical neoplasia and cancer | journal = CA: a cancer journal for clinicians | volume = 52 | issue = 6 | pages = 342–62 | year = 2002 | pmid = 12469763 | doi = 10.3322/canjclin.52.6.342 }}</ref><ref>{{cite journal | author = Strander B | title = At what age should cervical screening stop? | journal = Brit Med J | volume = 338 | issue = | pages = b809| year = 2009 | pmid = 19395422| doi = 10.1136/bmj.b809 }}</ref> |
||
Revision as of 01:17, 25 September 2013
| Cervical cancer | |
|---|---|
| Specialty | Oncology |
Cervical cancer is a malignant neoplasm arising from cells originating in the cervix uteri. One of the most common symptoms of cervical cancer is abnormal vaginal bleeding, but in some cases there may be no obvious symptoms until the cancer has progressed to an advanced stage.[1] Treatment usually consists of surgery (including local excision) in early stages, and chemotherapy and/or radiotherapy in more advanced stages of the disease.
Cancer screening using the Pap smear can identify precancerous and potentially precancerous changes in cervical cells and tissue. Treatment of high-grade changes can prevent the development of cancer in many victims. In developed countries, the widespread use of cervical screening programs has dramatically reduced the incidence of invasive cervical cancer.[2]
Human papillomavirus (HPV) infection appears to be a necessary factor in the development of almost all cases (90+%) of cervical cancer.[1][3] HPV vaccines effective against the two strains of this large family of viruses that currently cause approximately 70% of cases of cervical cancer have been licensed in the U.S, Canada, Australia, and the EU.[4][5] Since the vaccines only cover some of the cancer-causing ("high-risk") types of HPV, women should seek regular Pap smear screening, even after vaccination.[6]
The cervix is the narrow portion of the uterus where it joins with the top of the vagina. Most cervical cancers are squamous cell carcinomas, arising in the squamous (flattened) epithelial cells that line the cervix. Adenocarcinoma, arising in glandular epithelial cells is the second most common type. Very rarely, cancer can arise in other types of cells in the cervix.
Signs and symptoms
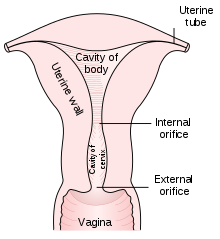
The early stages of cervical cancer may be completely asymptomatic.[1][2] Vaginal bleeding, contact bleeding, or (rarely) a vaginal mass may indicate the presence of malignancy. Also, moderate pain during sexual intercourse and vaginal discharge are symptoms of cervical cancer. In advanced disease, metastases may be present in the abdomen, lungs or elsewhere.
Symptoms of advanced cervical cancer may include: loss of appetite, weight loss, fatigue, pelvic pain, back pain, leg pain, swollen legs, heavy bleeding from the vagina, bone fractures, and/or (rarely) leakage of urine or faeces from the vagina.[7]
Causes
Infection with some types of human papilloma virus (HPV) is the greatest risk factor for cervical cancer, followed by smoking.[8] Other risk factors include human immunodeficiency virus.[8] Not all of the causes of cervical cancer are known, however, and several other contributing factors have been implicated.[9]
Human papillomavirus
Human papillomavirus type 16 and 18 are the cause of 75% of cervical cancer globally while 31 and 45 are the cause of another 10%.[10][11]
Women who have many sexual partners (or who have sex with men who have had many other partners) have a greater risk.[12][13]
Of the 150-200 types of HPV known,[14][15] 15 are classified as high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82), 3 as probable high-risk (26, 53, and 66), and 12 as low-risk (6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108).[16]
Genital warts, which are a form of benign tumor of epithelial cells, are also caused by various strains of HPV. However, these serotypes are usually not related to cervical cancer. It is common to have multiple strains at the same time, including those that can cause cervical cancer along with those that cause warts. The medically accepted paradigm, officially endorsed by the American Cancer Society and other organizations, is that a patient must have been infected with HPV to develop cervical cancer, and is hence viewed as a sexually transmitted disease (although many dispute that, technically, it is the causative agent, not the cancer, that is a sexually transmitted disease), but most women infected with high risk HPV will not develop cervical cancer.[17] Use of condoms reduces, but does not always prevent transmission. Likewise, HPV can be transmitted by skin-to-skin-contact with infected areas. In males, there is no commercially available test for HPV, although HPV is thought to grow preferentially in the epithelium of the glans penis, and cleaning of this area may be preventative.[citation needed]
Smoking
Smoking has also been linked to the development of cervical cancer.[18][19][20] There are a few different ways that smoking can increase the risk of cervical cancer in women which can be by direct and indirect methods of inducing cervical cancer.[18][20][21] A direct way of contracting this cancer is a female smoker has a higher chance of CIN3 occurring which has the potential of forming cervical cancer.[18] When CIN3 lesions lead to cancer, most of them have the assistance of the HPV virus, but that is not always the case which is why it can be considered a direct link to cervical cancer.[21] An indirect means of developing this cancer by smoking is that it can lead to human papillomavirus which can result in cervical cancer.[19] Heavy smoking and long term smoking seem to have more of a risk of getting the CIN3 lesions than lighter smoking or not smoking at all.[22] Although smoking has been linked to cervical cancer, it aids in the development of HPV which is the leading cause of this type of cancer.[20] Also, not only does it aid in the development of HPV, but if the woman is already HPV-positive she is at an even greater likelihood of contracting cervical cancer. [22]
Diagnosis

Biopsy
While the pap smear is an effective screening test, confirmation of the diagnosis of cervical cancer or pre-cancer requires a biopsy of the cervix. This is often done through colposcopy, a magnified visual inspection of the cervix aided by using a dilute acetic acid (e.g. vinegar) solution to highlight abnormal cells on the surface of the cervix.[1] Medical devices used for biopsy of the cervix include punch forceps, SpiraBrush CX, SoftBiopsy or Soft-ECC.
Colposcopic impression, the estimate of disease severity based on the visual inspection, forms part of the diagnosis.
Further diagnostic and treatment procedures are loop electrical excision procedure (LEEP) and conization, in which the inner lining of the cervix is removed to be examined pathologically. These are carried out if the biopsy confirms severe cervical intraepithelial neoplasia.


Precancerous lesions
Cervical intraepithelial neoplasia, the potential precursor to cervical cancer, is often diagnosed on examination of cervical biopsies by a pathologist. For premalignant dysplastic changes, the CIN (cervical intraepithelial neoplasia) grading is used.
The naming and histologic classification of cervical carcinoma percursor lesions has changed many times over the 20th century. The World Health Organization classification[23][24] system was descriptive of the lesions, naming them mild, moderate or severe dysplasia or carcinoma in situ (CIS). The term, Cervical Intraepithelial Neoplasia (CIN) was developed to place emphasis on the spectrum of abnormality in these lesions, and to help standardise treatment.[24] It classifies mild dysplasia as CIN1, moderate dysplasia as CIN2, and severe dysplasia and CIS as CIN3. More recently, CIN2 and CIN3 have been combined into CIN2/3. These results are what a pathologist might report from a biopsy.
These should not be confused with the Bethesda System terms for Pap smear (cytopathology) results. Among the Bethesda results: Low-grade Squamous Intraepithelial Lesion (LSIL) and High-grade Squamous Intraepithelial Lesion (HSIL). An LSIL Pap may correspond to CIN1, and HSIL may correspond to CIN2 and CIN3,[24] however they are results of different tests, and the Pap smear results need not match the histologic findings.
Cancer subtypes
Histologic subtypes of invasive cervical carcinoma include the following:[25][26] Though squamous cell carcinoma is the cervical cancer with the most incidence, the incidence of adenocarcinoma of the cervix has been increasing in recent decades.[1]
- squamous cell carcinoma (about 80-85%[citation needed])
- adenocarcinoma (about 15% of cervical cancers in the UK[23])
- adenosquamous carcinoma
- small cell carcinoma
- neuroendocrine tumour
- glassy cell carcinoma
- villoglandular adenocarcinoma
Non-carcinoma malignancies which can rarely occur in the cervix include
Note that the FIGO stage does not incorporate lymph node involvement in contrast to the TNM staging for most other cancers.
For cases treated surgically, information obtained from the pathologist can be used in assigning a separate pathologic stage but is not to replace the original clinical stage.
Staging
Cervical cancer is staged by the International Federation of Gynecology and Obstetrics (FIGO) staging system, which is based on clinical examination, rather than surgical findings. It allows only the following diagnostic tests to be used in determining the stage: palpation, inspection, colposcopy, endocervical curettage, hysteroscopy, cystoscopy, proctoscopy, intravenous urography, and X-ray examination of the lungs and skeleton, and cervical conization.
Prevention
Screening
The widespread introduction of cervical screening by the Papanicolaou test, or Pap smear for cervical cancer has been credited with dramatically reducing the incidence and mortality of cervical cancer in developed countries.[2] Pap smear screening every 3–5 years with appropriate follow-up can reduce cervical cancer incidence by up to 80%.[27] Abnormal results may suggest the presence of pre cancerous changes allowing examination and possible preventive treatment. If precancerous disease or cervical cancer is detected early, it can be monitored or treated relatively noninvasively, with little impairment of fertility.[citation needed] Personal invitations encouraging women to get screened are effective at increasing the likelihood they will do so. Educational materials also help increase the likelihood women will go for screening, but they are not as effective as invitations.[28]
Cervical cancer screening is typically recommended starting at age 21.[29][30] Recommendations for how often a Pap smear should be done vary from once a year to once every five years, in the absence of abnormal results.[27] Guidelines vary on how long to continue screening, but well screened women who have not had abnormal smears can stop screening about age 60 to 70.[31][32][33]
Liquid-based cytology is another potential screening method.[34][35] Although it was probably intended to improve on the accuracy of the Pap test, its main advantage has been to reduce the number of inadequate smears from around 9% to around 1%.[36] This reduces the need to recall women for a further smear. The United States Preventive Services Task Force supports screening every 5 years in those who are between 30 and 65 years when cytology is used in combination with HPV testing.[37]
Vaccination
There are two HPV vaccines (Gardasil and Cervarix) which reduce the risk of cancerous or precancerous changes of the cervix and perineum by about 93%.[38]
HPV vaccines are typically given to women age 9 to 26 as the vaccine is only effective if given before infection occurs. The vaccines have been shown to be effective for at least 4[6] to 6[39] years, and it is believed they will be effective for longer;[40] however, the duration of effectiveness and whether a booster will be needed is unknown. The high cost of this vaccine has been a cause for concern. Several countries have considered (or are considering) programs to fund HPV vaccination.
Condoms
Condoms are thought to offer some protection against cervical cancer.[41] Evidence on whether condoms protect against HPV infection is mixed, but they may protect against genital warts and the precursors to cervical cancer.[41] They also provide protection against other STDs, such as HIV and Chlamydia, which are associated with greater risks of developing cervical cancer.
Condoms may also be useful in treating potentially precancerous changes in the cervix. Exposure to semen appears to increase the risk of precancerous changes (CIN 3), and use of condoms helps to cause these changes to regress and helps clear HPV.[42] One study suggests that prostaglandin in semen may fuel the growth of cervical and uterine tumours and that affected women may benefit from the use of condoms.[43][44]
Nutrition
Vitamin A is associated with a lower risk[45] as is vitamin B12, vitamin C, vitamin E, and beta-carotene.[46]
Treatment
The treatment of cervical cancer varies worldwide, largely due to large variances in disease burden in developed and developing nations, access to surgeons skilled in radical pelvic surgery, and the emergence of "fertility sparing therapy" in developed nations. Because cervical cancers are radiosensitive, radiation may be used in all stages where surgical options do not exist.
Microinvasive cancer (stage IA) may be treated by hysterectomy (removal of the whole uterus including part of the vagina). For stage IA2, the lymph nodes are removed as well. Alternatives include local surgical procedures such as a loop electrical excision procedure (LEEP) or cone biopsy.[47] For 1A1 disease, a cone biopsy (aka cervical conization) is considered curative.
If a cone biopsy does not produce clear margins[48] (findings on biopsy showing that the tumor is surrounded by cancer free tissue, suggesting all of the tumor is removed), one more possible treatment option for patients who want to preserve their fertility is a trachelectomy.[49] This attempts to surgically remove the cancer while preserving the ovaries and uterus, providing for a more conservative operation than a hysterectomy. It is a viable option for those in stage I cervical cancer which has not spread; however, it is not yet considered a standard of care,[50] as few doctors are skilled in this procedure. Even the most experienced surgeon cannot promise that a trachelectomy can be performed until after surgical microscopic examination, as the extent of the spread of cancer is unknown. If the surgeon is not able to microscopically confirm clear margins of cervical tissue once the patient is under general anesthesia in the operating room, a hysterectomy may still be needed. This can only be done during the same operation if the patient has given prior consent. Due to the possible risk of cancer spread to the lymph nodes in stage 1b cancers and some stage 1a cancers, the surgeon may also need to remove some lymph nodes from around the uterus for pathologic evaluation.
A radical trachelectomy can be performed abdominally[51] or vaginally[52] and there are conflicting opinions as to which is better.[53] A radical abdominal trachelectomy with lymphadenectomy usually only requires a two to three day hospital stay, and most women recover very quickly (approximately six weeks). Complications are uncommon, although women who are able to conceive after surgery are susceptible to preterm labor and possible late miscarriage.[54] It is generally recommended to wait at least one year before attempting to become pregnant after surgery.[55] Recurrence in the residual cervix is very rare if the cancer has been cleared with the trachelectomy.[50] Yet, it is recommended for patients to practice vigilant prevention and follow up care including pap screenings/colposcopy, with biopsies of the remaining lower uterine segment as needed (every 3–4 months for at least 5 years) to monitor for any recurrence in addition to minimizing any new exposures to HPV through safe sex practices until one is actively trying to conceive.
Early stages (IB1 and IIA less than 4 cm) can be treated with radical hysterectomy with removal of the lymph nodes or radiation therapy. Radiation therapy is given as external beam radiotherapy to the pelvis and brachytherapy (internal radiation). Patients treated with surgery who have high risk features found on pathologic examination are given radiation therapy with or without chemotherapy in order to reduce the risk of relapse.
Larger early stage tumors (IB2 and IIA more than 4 cm) may be treated with radiation therapy and cisplatin-based chemotherapy, hysterectomy (which then usually requires adjuvant radiation therapy), or cisplatin chemotherapy followed by hysterectomy.
Advanced stage tumors (IIB-IVA) are treated with radiation therapy and cisplatin-based chemotherapy.
On June 15, 2006, the US Food and Drug Administration approved the use of a combination of two chemotherapy drugs, hycamtin and cisplatin for women with late-stage (IVB) cervical cancer treatment.[56] Combination treatment has significant risk of neutropenia, anemia, and thrombocytopenia side effects. Hycamtin is manufactured by GlaxoSmithKline.
Prognosis
Prognosis depends on the stage of the cancer. With treatment, the 5-year relative survival rate for the earliest stage of invasive cervical cancer is 92%, and the overall (all stages combined) 5-year survival rate is about 72%. These statistics may be improved when applied to women newly diagnosed, bearing in mind that these outcomes may be partly based on the state of treatment five years ago when the women studied were first diagnosed.[57]
With treatment, 80 to 90% of women with stage I cancer and 60 to 75% of those with stage II cancer are alive 5 years after diagnosis. Survival rates decrease to 30 to 40% for women with stage III cancer and 15% or fewer of those with stage IV cancer 5 years after diagnosis.[58]
According to the International Federation of Gynecology and Obstetrics, survival improves when radiotherapy is combined with cisplatin-based chemotherapy.[59]
As the cancer metastasizes to other parts of the body, prognosis drops dramatically because treatment of local lesions is generally more effective than whole body treatments such as chemotherapy.
Interval evaluation of the patient after therapy is imperative. Recurrent cervical cancer detected at its earliest stages might be successfully treated with surgery, radiation, chemotherapy, or a combination of the three. Thirty-five percent of patients with invasive cervical cancer have persistent or recurrent disease after treatment.[60]
Average years of potential life lost from cervical cancer are 25.3 (SEER Cancer Statistics Review 1975-2000, National Cancer Institute (NCI)). Approximately 4,600 women were projected to die in 2001 in the US of cervical cancer (DSTD), and the annual incidence was 13,000 in 2002 in the US, as calculated by SEER. Thus the ratio of deaths to incidence is approximately 35.4%.
Regular screening has meant that pre cancerous changes and early stage cervical cancers have been detected and treated early. Figures suggest that cervical screening is saving 5,000 lives each year in the UK by preventing cervical cancer.[61] About 1,000 women per year die of cervical cancer in the UK.
Epidemiology

Worldwide, cervical cancer is second most common[63] and the fifth deadliest cancer in women.[64] It affects about 16 per 100,000 women per year and kills about 9 per 100,000 per year.[65] Approximately 80% of cervical cancers occur in developing countries[66] Worldwide, in 2008, it was estimated that there were 473,000 cases of cervical cancer,[67] and in 2010 225,000 deaths.[68]
In the United States, it is only the 8th most common cancer of women. The median age at diagnosis is 48. Hispanic women are significantly more likely to be diagnosed with cervical cancer than the general population.[69] In 1998, about 12,800 women were diagnosed in the US and about 4,800 died.[2] In 2008 in the US an estimated 11,000 new cases were expected to be diagnosed, and about 3,870 were expected to die of cervical cancer.[57] Among gynecological cancers it ranks behind endometrial cancer and ovarian cancer. The incidence and mortality in the US are about half those for the rest of the world, which is due in part to the success of screening with the Pap smear.[2] The incidence of new cases of cervical cancer in the United States was 7 per 100,000 women in 2004.[70] Cervical cancer deaths decreased by approximately 74% in the last 50 years, largely due to widespread Pap smear screening.[63] The annual direct medical cost of cervical cancer prevention and treatment prior to introduction of the HPV vaccine was estimated at $6 billion.[63]
In the European Union, there were about 34,000 new cases per year and over 16,000 deaths due to cervical cancer in 2004.[27]
In the United Kingdom, the age-standardised (European) incidence is 8.5/100,000 per year (2006). It is the twelfth most common cancer in women, accounting for 2% of all female cancers, and is the second most common cancer in the under 35s females, after breast cancer. The UK's European age-standardised mortality is 2.4/100,000 per year (2007) (Cancer Research UK Cervical cancer statistics for the UK).[71] With a 42% reduction from 1988-1997 the NHS implemented screening programme has been highly successful, screening the highest risk age group (25–49 years) every 3 years, and those ages 50–64 every 5 years.
In Canada, an estimated 1,300 women will be diagnosed with cervical cancer in 2008 and 380 will die.[72]
In Australia, there were 734 cases of cervical cancer (2005). The number of women diagnosed with cervical cancer has dropped on average by 4.5% each year since organised screening began in 1991 (1991–2005).[73] Regular two-yearly Pap tests can reduce the incidence of cervical cancer by up to 90% in Australia, and save 1,200 Australian women dying from the disease each year.[74]
History
- 400 BCE - Hippocrates: cervical cancer incurable
- 1925 - Hinselmann: invented colposcope
- 1928 - Papanicolaou: developed Papanicolaou technique
- 1941 - Papanicolaou and Trout: Pap smear screening
- 1946 - Ayer: Aylesbury spatula to scrape the cervix, collecting sample for Pap smear
- 1951 - First successful in-vitro cell line, HeLa, derived from biopsy of cervical cancer of Henrietta Lacks
- 1976 - Zur Hausen and Gisam: found HPV DNA in cervical cancer and warts
- 1988 - Bethesda System for reporting Pap results developed
- 2006 - First HPV vaccine FDA approved
Epidemiologists working in the early 20th century noted that cervical cancer behaved like a sexually transmitted disease. In summary:
- Cervical cancer was common in female sex workers.
- It was rare in nuns, except for those who had been sexually active before entering the convent. (Rigoni in 1841)
- It was more common in the second wives of men whose first wives had died from cervical cancer.
- It was rare in Jewish women.[75]
- In 1935, Syverton and Berry discovered a relationship between RPV (Rabbit Papillomavirus) and skin cancer in rabbits. (HPV is species-specific and therefore cannot be transmitted to rabbits)
This led to the suspicion that cervical cancer could be caused by a sexually transmitted agent. Initial research in the 1940s and 1950s put the blame on smegma (e.g. Heins et al. 1958).[76] During the 1960s and 1970s it was suspected that infection with herpes simplex virus was the cause of the disease. In summary, HSV was seen as a likely cause[77] because it is known to survive in the female reproductive tract, to be transmitted sexually in a way compatible with known risk factors, such as promiscuity and low socioeconomic status. Herpes viruses were also implicated in other malignant diseases, including Burkitt's lymphoma, Nasopharyngeal carcinoma, Marek's disease and the Lucké renal adenocarcinoma. HSV was recovered from cervical tumour cells.
A description of human papillomavirus (HPV) by electron microscopy was given in 1949, and HPV-DNA was identified in 1963.[citation needed] It was not until the 1980s that HPV was identified in cervical cancer tissue.[78] It has since been demonstrated that HPV is implicated in virtually all cervical cancers.[5] Specific viral subtypes implicated are HPV 16, 18, 31, 45 and others.
In work that was initiated in the mid 1980s, the HPV vaccine was developed, in parallel, by researchers at Georgetown University Medical Center, the University of Rochester, the University of Queensland in Australia, and the U.S. National Cancer Institute.[79] In 2006, the U.S. Food and Drug Administration (FDA) approved the first preventive HPV vaccine, marketed by Merck & Co. under the trade name Gardasil.
Society and culture
Australia
In Australia, Aboriginal women are more than five times more likely to die from cervical cancer than non-Aboriginal women, suggesting that Aboriginal women are less likely to have regular Pap tests.[80] There are several factors that may limit indigenous women from engaging in regular cervical screening practices, including sensitivity in discussing the topic in Aboriginal communities, embarrassment, anxiety and fear about the procedure.[81] Difficulty in accessing screening services (for example, transport difficulties) and a lack of female GPs, trained pap smear providers and trained female Aboriginal Health Workers are also issues.[81]
The Australian Cervical Cancer Foundation (ACCF) was established in 2008 with the vision 'to protect and enhance women’s health by eliminating cervical cancer and enabling treatment for women with cervical cancer and related health issues, in Australia and in developing countries.'[82] Ian Frazer, one of the developers of the Gardasil cervical cancer vaccine, is the scientific advisor to ACCF.[83]
Janette Howard, the wife of former Australian Prime Minister John Howard, was diagnosed with cervical cancer in 1996, and first spoke on her battle with the disease in 2006.[84] Many people had assumed Mrs Howard had suffered from breast cancer.[84]
Japan
Since 2010, girls in Japan have been eligible to receive the cervical cancer vaccination for free.[85] In June 2013, the Japanese Ministry of Health, Labor and Welfare mandated that, before administering the vaccine, medical institutions must inform girls that the Ministry does not recommend it.[85] However, the vaccine is still available at no cost to Japanese girls who choose to accept the vaccination.[85]
Nordic countries
All of the Nordic countries have cervical cancer screening programs in place.[86] Pap smear was integrated into clinical practice in the Nordic countries in the 1960s.[86]
United States of America
A 2007 survey of 3,076 American women found only 40% had heard of HPV infection and less than half of those knew it causes cervical cancer.[87]
References
- ^ a b c d e Kumar V, Abbas AK, Fausto N, Mitchell RN (2007). Robbins Basic Pathology ((8th ed.) ed.). Saunders Elsevier. pp. 718–721. ISBN 978-1-4160-2973-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Canavan TP, Doshi NR (2000). "Cervical cancer". Am Fam Physician. 61 (5): 1369–76. PMID 10735343.
- ^ Walboomers JM, Jacobs MV, Manos MM; et al. (1999). "Human papillomavirus is a necessary cause of invasive cervical cancer worldwide". J. Pathol. 189 (1): 12–9. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. PMID 10451482.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "FDA Licenses New Vaccine for Prevention of Cervical Cancer". U.S. Food and Drug Administration. 2006-06-08. Retrieved 2007-12-02.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b Lowy DR, Schiller JT (2006). "Prophylactic human papillomavirus vaccines". J. Clin. Invest. 116 (5): 1167–73. doi:10.1172/JCI28607. PMC 1451224. PMID 16670757.
- ^ a b "Human Papillomavirus (HPV) Vaccines: Q & A". National Cancer Institute. Retrieved 2008-07-18. Cite error: The named reference "National Cancer Institute HPV Q&A" was defined multiple times with different content (see the help page).
- ^ Nanda, Rita (2006-06-09). "Cervical cancer". MedlinePlus Medical Encyclopedia. National Institutes of Health. Retrieved 2007-12-02.
- ^ a b Gadducci A, Barsotti, C, Cosio, S, Domenici, L, Riccardo Genazzani, A (2011). "Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature". Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 27 (8): 597–604. doi:10.3109/09513590.2011.558953. PMID 21438669.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Stuart Campbell (2006). Gynaecology by Ten Teachers (18 ed.). Hodder Education. ISBN 0-340-81662-7.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Dillman, edited by Robert K. Oldham, Robert O. (2009). Principles of cancer biotherapy (5th ed.). Dordrecht: Springer. p. 149. ISBN 9789048122899.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N (1999). "Human papillomavirus is a necessary cause of invasive cervical cancer worldwide". J. Pathol. 189 (1): 12–9. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. PMID 10451482.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "What Causes Cancer of the Cervix?". American Cancer Society. 2006-11-30. Archived from the original on 2007-10-13. Retrieved 2007-12-02.
- ^ Marrazzo JM, Koutsky LA, Kiviat NB, Kuypers JM, Stine K (2001). "Papanicolaou test screening and prevalence of genital human papillomavirus among women who have sex with women". Am J Public Health. 91 (6): 947–52. doi:10.2105/AJPH.91.6.947. PMC 1446473. PMID 11392939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "HPV Type-Detect". Medical Diagnostic Laboratories. 2007-10-30. Archived from the original on 2007-09-27. Retrieved 2007-12-02.
- ^ Gottlieb, Nicole (2002-04-24). "A Primer on HPV". Benchmarks. National Cancer Institute. Retrieved 2007-12-02.
- ^ Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ (2003). "Epidemiologic classification of human papillomavirus types associated with cervical cancer". N. Engl. J. Med. 348 (6): 518–27. doi:10.1056/NEJMoa021641. PMID 12571259.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ (2006). "HPV-mediated cervical carcinogenesis: concepts and clinical implications". J. Pathol. 208 (2): 152–64. doi:10.1002/path.1866. PMID 16362994.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Luhn, Patricia; Walker, Joan; Schiffman, Mark; Zuna, Rosemary E.; Dunn, S. Terence; Gold, Michael A.; Smith, Katherine; Mathews, Cara; Allen, Richard A.; Zhang, Roy; Wang, Sophia; Wentzensen, Nicolas (2013). "The role of co-factors in the progression from human papillomavirus infection to cervical cancer". Gynecologic Oncology. 128 (2): 265–270. doi:10.1016/j.ygyno.2012.11.003. ISSN 0090-8258. PMID 23146688.
- ^ a b Remschmidt, Cornelius; Kaufmann, Andreas M.; Hagemann, Ingke; Vartazarova, Elena; Wichmann, Ole; Deleré, Yvonne (2013). "Risk Factors for Cervical Human Papillomavirus Infection and High-Grade Intraepithelial Lesion in Women Aged 20 to 31 Years in Germany". International Journal of Gynecological Cancer. 23 (3): 519–526. doi:10.1097/IGC.0b013e318285a4b2. ISSN 1048-891X. PMID 23360813.
- ^ a b c Gadducci, Angiolo; Barsotti, Cecilia; Cosio, Stefania; Domenici, Lavinia; Riccardo Genazzani, Andrea (2011). "Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature". Gynecological Endocrinology. 27 (8): 597–604. doi:10.3109/09513590.2011.558953. ISSN 0951-3590. PMID 21438669.
- ^ a b Agorastos, Theodoros; Miliaras, Dimosthenis; Lambropoulos, Alexandros F.; Chrisafi, Sophia; Kotsis, Alexandros; Manthos, Anastasios; Bontis, John (2005). "Detection and typing of human papillomavirus DNA in uterine cervices with coexistent grade I and grade III intraepithelial neoplasia: biologic progression or independent lesions?". European Journal of Obstetrics & Gynecology and Reproductive Biology. 121 (1): 99–103. doi:10.1016/j.ejogrb.2004.11.024. ISSN 0301-2115.
- ^ a b Jensen, K. E.; Schmiedel, S.; Frederiksen, K.; Norrild, B.; Iftner, T.; Kjaer, S. K. (2012). "Risk for Cervical Intraepithelial Neoplasia Grade 3 or Worse in Relation to Smoking among Women with Persistent Human Papillomavirus Infection". Cancer Epidemiology Biomarkers & Prevention. 21 (11): 1949–1955. doi:10.1158/1055-9965.EPI-12-0663. ISSN 1055-9965.
- ^ a b "Cancer Research UK website". Retrieved 2009-01-03.
- ^ a b c DeMay, M (2007). Practical principles of cytopathology. Revised edition. Chicago, IL: American Society for Clinical Pathology Press. ISBN 978-0-89189-549-7.
- ^ Garcia A Hamid O, El-Khoueiry A (2006-07-06). "Cervical Cancer". eMedicine. WebMD. Retrieved 2007-12-02.
- ^ Dolinsky, Christopher (2006-07-17). "Cervical Cancer: The Basics". OncoLink. Abramson Cancer Center of the University of Pennsylvania. Retrieved 2007-12-02.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b c Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L (2010). "European Guidelines for Quality Assurance in Cervical Cancer Screening. Second Edition—Summary Document". Annals of Oncology. 21 (3): 448–458. doi:10.1093/annonc/mdp471. PMC 2826099. PMID 20176693.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Everett, T.; Bryant, A.; Griffin, MF.; Martin-Hirsch, PP.; Forbes, CA.; Jepson, RG. (2011). "Interventions targeted at women to encourage the uptake of cervical screening". Cochrane Database Syst Rev (5): CD002834. doi:10.1002/14651858.CD002834.pub2. PMID 21563135.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Saslow D, Solomon, D, Lawson, HW, Killackey, M, Kulasingam, SL, Cain, J, Garcia, FA, Moriarty, AT, Waxman, AG, Wilbur, DC, Wentzensen, N, Downs LS, Jr, Spitzer, M, Moscicki, AB, Franco, EL, Stoler, MH, Schiffman, M, Castle, PE, Myers, ER (2012). "American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer". CA: a cancer journal for clinicians. 62 (3): 147–72. doi:10.3322/caac.21139. PMID 22422631.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moyer VA, on behalf of the U.S. Preventive Services Task, Force (2012). "Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement". Annals of internal medicine. doi:10.1059/0003-4819-156-12-201206190-00424. PMID 22422943.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ U.S. Preventive Services Task Force (2003). "Screening for Cervical Cancer: Recommendations and Rationale. AHRQ Publication No. 03-515A". Rockville, MD.: Agency for Healthcare Research and Quality. Retrieved June 5, 2010..
{{cite web}}: Check date values in:|accessdate=(help) - ^ Saslow D, Runowicz CD, Solomon D; et al. (2002). "American Cancer Society guideline for the early detection of cervical neoplasia and cancer". CA: a cancer journal for clinicians. 52 (6): 342–62. doi:10.3322/canjclin.52.6.342. PMID 12469763.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Strander B (2009). "At what age should cervical screening stop?". Brit Med J. 338: b809. doi:10.1136/bmj.b809. PMID 19395422.
- ^ Payne N, Chilcott J, McGoogan E (2000). "Liquid-based cytology in cervical screening: a rapid and systematic review". Health technology assessment (Winchester, England). 4 (18): 1–73. PMID 10932023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Karnon, J (2004 May). "Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis". Health technology assessment (Winchester, England). 8 (20): iii, 1–78. PMID 15147611.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Liquid Based Cytology (LBC): NHS Cervical Screening Programme". Retrieved 2010-10-01.
- ^ Moyer, VA; U.S. Preventive Services Task Force (2012 Jun 19). "Screening for cervical cancer: u.s. Preventive services task force recommendation statement". Annals of internal medicine. 156 (12): 880–91. doi:10.7326/0003-4819-156-12-201206190-00424. PMID 22711081.
{{cite journal}}: Check date values in:|date=(help) - ^ Medeiros LR, Rosa, DD, da Rosa, MI, Bozzetti, MC, Zanini, RR (2009). "Efficacy of human papillomavirus vaccines: a systematic quantitative review". International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 19 (7): 1166–76. doi:10.1111/IGC.0b013e3181a3d100. PMID 19823051.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Harper D, Gall S, Naud P, Quint W, Dubin G, Jenkins D; et al. (2008). "Sustained immunogenicity and high efficacy against HPV 16/18 related cervical neoplasia: Long-term follow up through 6.4 years in women vaccinated with Cervarix (GSK's HPV-16/18 AS04 candidate vaccine)". Gynecol Oncol. 109: 158–159. doi:10.1016/j.ygyno.2008.02.017.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ &Na; (2010). "Committee opinion no. 467: human papillomavirus vaccination". Obstet Gynecol. 116 (3): 800–3. doi:10.1097/AOG.0b013e3181f680c8. PMID 20733476.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ a b Manhart LE, Koutsky LA (2002). "Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis". Sex Transm Dis. 29 (11): 725–35. doi:10.1097/00007435-200211000-00018. PMID 12438912.
- ^ Cornelis J.A. Hogewoning, Maaike C.G. Bleeker; et al. (2003). "Condom use Promotes the Regression of Cervical Intraepithelial Neoplasia and Clearance of HPV: Randomized Clinical Trial". International Journal of Cancer. 107 (5): 811–816. doi:10.1002/ijc.11474. PMID 14566832.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "Semen 'may fuel cervical cancer'". BBC. 2006-08-31. Retrieved 2007-12-02.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ [http ://www.mrc.ac.uk/NewsViewsAndEvents/News/MRC002621 "Semen can worsen cervical cancer"]. Medical Research Council (UK). Retrieved 2007-12-02.
{{cite news}}: Check|url=value (help); Cite has empty unknown parameter:|coauthors=(help) - ^ Zhang X, Dai, B, Zhang, B, Wang, Z (2011). "Vitamin A and risk of cervical cancer: A meta-analysis". Gynecologic oncology. 124 (2): 366–73. doi:10.1016/j.ygyno.2011.10.012. PMID 22005522.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Myung SK, Ju, W, Kim, SC, Kim, H, Korean Meta-analysis (KORMA) Study, Group (2011). "Vitamin or antioxidant intake (or serum level) and risk of cervical neoplasm: a meta-analysis". BJOG : an international journal of obstetrics and gynaecology. 118 (11): 1285–91. doi:10.1111/j.1471-0528.2011.03032.x. PMID 21749626.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Erstad, Shannon (2007-01-12). "Cone biopsy (conization) for abnormal cervical cell changes". WebMD. Retrieved 2007-12-02.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Jones WB, Mercer GO, Lewis JL, Rubin SC, Hoskins WJ (1993). "Early invasive carcinoma of the cervix". Gynecol. Oncol. 51 (1): 26–32. doi:10.1006/gyno.1993.1241. PMID 8244170.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dolson, Laura (2001). "Trachelectomy". Retrieved 2007-12-02.
- ^ a b Burnett AF (2006). "Radical trachelectomy with laparoscopic lymphadenectomy: review of oncologic and obstetrical outcomes". Curr. Opin. Obstet. Gynecol. 18 (1): 8–13. doi:10.1097/01.gco.0000192968.75190.dc. PMID 16493253.
- ^ Cibula D, Ungár L, Svárovský J, Zivný J, Freitag P (2005). "[Abdominal radical trachelectomy--technique and experience]". Ceska Gynekol (in Czech). 70 (2): 117–22. PMID 15918265.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Plante M, Renaud MC, Hoskins IA, Roy M (2005). "Vaginal radical trachelectomy: a valuable fertility-preserving option in the management of early-stage cervical cancer. A series of 50 pregnancies and review of the literature". Gynecol. Oncol. 98 (1): 3–10. doi:10.1016/j.ygyno.2005.04.014. PMID 15936061.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Roy M, Plante M, Renaud MC, Têtu B (1996). "Vaginal radical hysterectomy versus abdominal radical hysterectomy in the treatment of early-stage cervical cancer". Gynecol. Oncol. 62 (3): 336–9. doi:10.1006/gyno.1996.0245. PMID 8812529.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dargent D, Martin X, Sacchetoni A, Mathevet P (2000). "Laparoscopic vaginal radical trachelectomy: a treatment to preserve the fertility of cervical carcinoma patients". Cancer. 88 (8): 1877–82. doi:10.1002/(SICI)1097-0142(20000415)88:8<1877::AID-CNCR17>3.0.CO;2-W. PMID 10760765.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schlaerth JB, Spirtos NM, Schlaerth AC (2003). "Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer". Am. J. Obstet. Gynecol. 188 (1): 29–34. doi:10.1067/mob.2003.124. PMID 12548192.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "FDA Approves First Drug Treatment for Late-Stage Cervical Cancer". U.S. Food and Drug Administration. 2006-06-15. Retrieved 2007-12-02.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b "What Are the Key Statistics About Cervical Cancer?". American Cancer Society. 2006-08-04. Archived from the original on 2007-10-30. Retrieved 2007-12-02. Cite error: The named reference "ACS Key Stats" was defined multiple times with different content (see the help page).
- ^ "Cervical Cancer". Cervical Cancer: Cancers of the Female Reproductive System: Merck Manual Home Edition. Merck Manual Home Edition. Retrieved 2007-03-24.
- ^ Committee on Practice Bulletins-Gynecology (2002). "ACOG practice bulletin. Diagnosis and treatment of cervical carcinomas, number 35, May 2002". Obstetrics and gynecology. 99 (5 Pt 1): 855–67. PMID 11978302.
- ^ "Cervical Cancer". Cervical Cancer: Pathology, Symptoms and Signs, Diagnosis, Prognosis and Treatment. Armenian Health Network, Health.am.
- ^ "Cervical cancer statistics and prognosis". Cancer Research UK. Retrieved 2007-03-24.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved Nov. 11, 2009.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b c Armstrong EP (2010). "Prophylaxis of Cervical Cancer and Related Cervical Disease: A Review of the Cost-Effectiveness of Vaccination Against Oncogenic HPV Types". Journal of Managed Care Pharmacy. 16 (3): 217–30. PMID 20331326.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ World Health Organization (2006). "Fact sheet No. 297: Cancer". Retrieved 2007-12-01.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "GLOBOCAN 2002 database: summary table by cancer". Archived from the original on 2008-06-16. Retrieved 2008-10-26.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20508781, please use {{cite journal}} with
|pmid=20508781instead. - ^ "NCCC National Cervical Cancer Coalition". Archived from the original on 2008-08-22. Retrieved 2008-07-01.
- ^ Lozano, R (2012 Dec 15). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. PMID 23245604.
{{cite journal}}: Check date values in:|date=(help) - ^ Howlader (November 10, 2011). "SEER Stat Fact Sheets: Cervix Uteri". National Cancer Institute. Retrieved 7 February 2012.
- ^ SEER cancer statistics
- ^ http://info.cancerresearchuk.org/cancerstats/types/cervix/mortality/
- ^ MacDonald N, Stanbrook MB, Hébert PC (2008). "Human papillomavirus vaccine risk and reality". CMAJ (in French). 179 (6): 503, 505. doi:10.1503/cmaj.081238. PMC 2527393. PMID 18762616.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Incidence and mortality rates".
- ^ http://www.papscreen.org.au/
- ^ Menczer J (2003). "The low incidence of cervical cancer in Jewish women: has the puzzle finally been solved?" (PDF). Isr. Med. Assoc. J. 5 (2): 120–3. PMID 12674663.
- ^ Heins Jr HC, EJ Dennis, HR Pratt-Thomas (1958). "The possible role of smegma in carcinoma of the cervix". American Journal of Obstetrics & Gynecology. 76 (4): 726–33. PMID 13583012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alexander ER, EJ Dennis, HR Pratt-Thomas (1973). "Possible Etiologies of Cancer of the Cervix Other Than Herpesvirus". Cancer Research. 33 (6): 1485–90. PMID 4352386.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dürst M, Gissmann L, Ikenberg H, zur Hausen H (1983). "A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions". Proceedings of the National Academy of Sciences of the United States of America. 80 (12): 3812–5. doi:10.1073/pnas.80.12.3812. PMC 394142. PMID 6304740.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^
McNeil C (2006). "Who invented the VLP cervical cancer vaccines?". J. Natl. Cancer Inst. 98 (7): 433. doi:10.1093/jnci/djj144. PMID 16595773.
{{cite journal}}: Unknown parameter|month=ignored (help) PMID 16595773 - ^ Cancer Institute NSW (2013). "Information about cervical screening for Aboriginal women". NSW Government. Archived from the original on 11 April 2013.
- ^ a b Mary-Anne Romano (17 October 2011). "Aboriginal cervical cancer rates parallel health inequity". Science Network Western Australia. Archived from the original on 14 May 2013.
- ^ Australin Cervical Cancer Foundation. "Vision and Mission". Australian Cervical Cancer Foundation. Archived from the original on 12 May 2013.
- ^ Australin Cervical Cancer Foundation. "Our People". Australian Cervical Cancer Foundation. Archived from the original on 12 May 2013.
- ^ a b Gillian Bradford (16 October 2006). "Janette Howard speaks on her battle with cervical cancer". Australian Broadcasting Corporation. Archived from the original on 13 November 2012.
{{cite web}}:|archive-date=/|archive-url=timestamp mismatch; 3 November 2012 suggested (help) - ^ a b c The Asahi Shimbun (15 June 2013). "Health ministry withdraws recommendation for cervical cancer vaccine". The Asahi Shimbun.
- ^ a b Mari Nygård (13 June 2011). "Screening for cervical cancer: when theory meets reality". BioMed Central.
- ^ Tiro JA, Meissner HI, Kobrin S, Chollette V (2007). "What do women in the U.S. know about human papillomavirus and cervical cancer?". Cancer Epidemiol. Biomarkers Prev. 16 (2): 288–94. doi:10.1158/1055-9965.EPI-06-0756. PMID 17267388.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G (2004). "Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial". Lancet. 364 (9447): 1757–65. doi:10.1016/S0140-6736(04)17398-4. PMID 15541448.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Peto J, C Gilham, O Fletcher, FE Matthews (2004). "The cervical cancer epidemic that screening has prevented in the UK". Lancet. 364 (9430): 249–56. doi:10.1016/S0140-6736(04)16674-9. PMID 15262102.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
