Niobium(V) fluoride
| |
| Names | |
|---|---|
| IUPAC names
Niobium(V) fluoride
Niobium pentafluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.109 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F5Nb | |
| Molar mass | 187.89839 g·mol−1 |
| Appearance | colorless hygroscopic solid |
| Density | 3.293 g/cm3 |
| Melting point | 72 to 73 °C (162 to 163 °F; 345 to 346 K) |
| Boiling point | 236 °C (457 °F; 509 K) |
| reacts | |
| Solubility | slightly soluble in chloroform, carbon disulfide, sulfuric acid |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H312, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Niobium(V) chloride Niobium(V) bromide Niobium(V) iodide |
Other cations
|
Vanadium(V) fluoride Tantalum(V) fluoride |
Related niobium fluorides
|
Niobium(III) fluoride Niobium(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
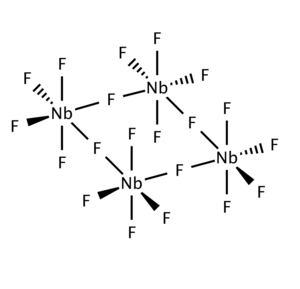
Niobium(V) fluoride, also known as niobium pentafluoride, is the inorganic compound with the formula NbF5. The solid consists of tetramers [NbF5]4. It is a colorless solid that is rarely used.[1]
Preparation and reactions
Niobium pentafluoride is obtained by treatment of any niobium compound with fluorine:[2]
- 2 Nb + 5 F2 → 2 NbF5
- 2 NbCl5 + 5 F2 → 2 NbF5 + 5 Cl2
It reacts with hydrogen fluoride to give H2NbF7, a superacid.
Related compounds
In hydrofluoric acid, NbF5 converts to [[NbF7]2- and [[NbF5O]2-. The relative solubility of these potassium salts and related tantalum fluorides are the basis of the Marignac process for separation of Nb and Ta.
NbCl5 forms a dimeric structure (edge-shared bioctahedron) in contrast to the corner-shared tetrameric structure of the fluoride.
External links
References
- ^ "Niobium and Niobium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a17_251.
{{cite encyclopedia}}: Cite uses deprecated parameter|authors=(help) - ^ Homer F. Priest (1950). "Anhydrous Metal Fluorides". Inorganic Syntheses. 3: 171. doi:10.1002/9780470132340.ch47.
{{cite journal}}: Cite has empty unknown parameter:|1=(help)
