Nothofagus
| Southern beeches Temporal range: Late Campanian–Present
| |
|---|---|

| |
| Nothofagus macrocarpa | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Fagales |
| Family: | Nothofagaceae Kuprian.[1] |
| Genus: | Nothofagus Blume |
| |
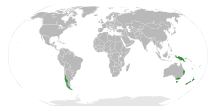
| The range of Nothofagus. | |
| Synonyms[2] | |
| |


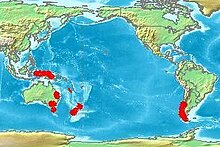
Nothofagus, also known as the southern beeches, is a genus of 43 species[3] of trees and shrubs native to the Southern Hemisphere in southern South America (Chile, Argentina) and Australasia (east and southeast Australia, New Zealand, New Guinea, and New Caledonia). The species are ecological dominants in many temperate forests in these regions.[4] Some species are reportedly naturalised in Germany and Great Britain.[2] The genus has a rich fossil record of leaves, cupules, and pollen, with fossils extending into the late Cretaceous period and occurring in Australia, New Zealand, Antarctica, and South America.[5] In the past, they were included in the family Fagaceae, but genetic tests revealed them to be genetically distinct,[6] and they are now included in their own family, the Nothofagaceae.[7]
The leaves are toothed or entire, evergreen or deciduous. The fruit is a small, flattened or triangular nut, borne in cupules containing one to seven nuts.
Nothofagus species are used as food plants by the larvae of hepialid moths of the genus Aenetus, including A. eximia and A. virescens.
Many individual trees are extremely old, and at one time, some populations were thought to be unable to reproduce in present-day conditions where they were growing, except by suckering (clonal reproduction), being remnant forest from a cooler time. Sexual reproduction has since been shown to be possible.[8] Although the genus now mostly occurs in cool, isolated, high-altitude environments at temperate and tropical latitudes, the fossil record shows that it survived in climates that appear to be much warmer than those that Nothofagus now occupies.[9]
Taxonomy
The genus Nothofagus was first formally described in 1850 by Carl Ludwig Blume who published the description in his book Museum botanicum Lugduno-Batavum, sive, Stirpium exoticarum novarum vel minus cognitarum ex vivis aut siccis brevis expositio et descriptio.[10][11]
Four sub-genera are recognized, based on morphology and DNA analysis:[12]
- Subgenus Fuscospora, six species (N. alessandri, N. cliffortioides, N. fusca, N. gunnii, N. solandri, and N. truncata) in New Zealand, Tasmania, and southern South America.
- Subgenus Lophozonia, seven species (N. alpina, N. cunninghamii, N. glauca, N. macrocarpa, N. menziesii, N. moorei, and N. obliqua) in New Zealand, Australia, and southern South America.
- Subgenus Nothofagus, five species (N. antarctica, N. betuloides, N. dombeyi, N. nitida, and N. pumilio) in southern South America.
- Subgenus Brassospora (or Trisyngyne), 25 species (N. aequilateralis, N. balansae, N. baumanniae, N. bernhardii, N. brassii, N. carrii, N. codonandra, N. cornuta, N. crenata, N. decipiens, N. discoidea, N. dura, N. eymae, N. flaviramea, N. grandis, N. nuda, N. perryi, N. pseudoresinosa, N, pullei, N. recurva, N. resinosa, N. rubra, N. starkenborghii, N. stylosa, and N. womersleyi) in New Guinea and New Caledonia.
Species list
The following is a list of species, hybrids and varieties accepted by the Plants of the World Online as at April 2020:[13]
- Nothofagus aequilateralis (Baum.-Bod.) Steenis (New Caledonia)
- Nothofagus alessandri Espinosa (Central Chile)
- Nothofagus alpina (Poepp. & Endl.) Oerst. (Argentina South, Chile Central, Chile South)
- Nothofagus antarctica (G.Forst.) Oerst. (Argentina South, Chile Central, Chile South)
- Nothofagus balansae (Baill.) Steenis (New Caledonia)
- Nothofagus baumanniae (Baum.-Bod.) Steenis (New Caledonia)
- Nothofagus betuloides (Mirb.) Oerst. (Argentina South, Chile South)
- Nothofagus brassii Steenis (New Guinea)
- Nothofagus carrii Steenis (New Guinea)
- Nothofagus cliffortioides (Hook.f.) Oerst. (New Zealand North, New Zealand South)
- Nothofagus codonandra (Hook.f.) Oerst. (New Caledonia)
- Nothofagus crenata Steenis(New Guinea)
- Nothofagus cunninghamii (Hook.f.) Oerst. (Tasmania, Victoria)
- Nothofagus discoidea (Baum.-Bod.) Steenis (New Caledonia)
- Nothofagus dombeyi (Mirb.) Oerst. (Argentina South, Chile Central, Chile South)
- Nothofagus flaviramea Steenis (New Guinea)
- Nothofagus fusca (Hook.f.) Oerst. (New Zealand North, New Zealand South)
- Nothofagus glauca (Phil.) Krasser (Chile Central)
- Nothofagus grandis Steenis (New Guinea)
- Nothofagus gunnii (Hook.f.) Oerst. (Tasmania)
- Nothofagus macrocarpa (A.DC.) F.M.Vázquez & R.A.Rodr. (Chile Central)
- Nothofagus menziesii (Hook.f.) Oerst. (New Zealand North, New Zealand South)
- Nothofagus moorei [F.Muell.) Krasser (New South Wales, Queensland)
- Nothofagus nitida (Phil.) Krasser (Chile South)
- Nothofagus nuda Steenis (New Guinea)
- Nothofagus obliqua (Mirb.) Oerst. (Argentina South, Chile Central, Chile South)
- Nothofagus perryi Steenis (New Guinea)
- Nothofagus pseudoresinosa Steenis (New Guinea)
- Nothofagus pullei Steenis (New Guinea)
- Nothofagus pumilio (Poepp. & Endl.) Krasser (Argentina South, Chile Central, Chile South)
- Nothofagus resinosa Steenis (New Guinea)
- Nothofagus rubra Steenis (New Guinea)
- Nothofagus rutila Ravenna (Chile Central)
- Nothofagus solandri (Hook.f.) Oerst. (New Zealand North, New Zealand South)
- Nothofagus starkenborghii Steenis (Bismarck Archipelago, New Guinea)
- Nothofagus stylosa Steenis (New Guinea)
- Nothofagus truncata (Colenso) Cockayne (New Zealand North, New Zealand South)
- Nothofagus womersleyi Steenis (New Guinea)
- Nothofagus × apiculata (Colenso) Cockayne (New Zealand North, New Zealand South)
- Nothofagus × blairii Kirk (New Zealand North, New Zealand South)
- Nothofagus × dodecaphleps Mike L.Grant & E.J.Clement
- Nothofagus × eugenananus Gilland.
- Nothofagus × leoni Espinosa (Chile Central)
- Nothofagus × solfusca Allan (New Zealand North)
- Nothofagus var. crenata Steenis (New Guinea)
- Nothofagus var. sapeii Steenis (New Guinea)
In 2013, Peter Brian Heenan and Rob D. Smissen proposed splitting the genus into four, turning the four recognized subgenera into the new genera Fuscospora, Lophozonia and Trisyngyne, with the five South American species of subgenus Nothofagus remaining in genus Nothofagus.[14] The proposed new genera are not accepted at the World Checklist of Selected Plant Families.[2][15]
Extinct species
The following additional species are listed as extinct:[5][16][17]
- Nothofagus balfourensis (Tasmania, Late Oligocene-Early Miocene)
- Nothofagus beardmorensis (Antarctica, either about 3 million or about 15 million years old)[18]
- Nothofagus bulbosa (Tasmania, Early Oligocene)
- Nothofagus cethanica (Tasmania, Early Oligocene)
- Nothofagus cooksoniae (Tasmania, Early Oligocene)
- Nothofagus lobata (Tasmania, Early Oligocene)
- Nothofagus mucronata (Tasmania, Early Oligocene)
- Nothofagus muelleri (New South Wales, Late Eocene)
- Nothofagus novae-zealandiae (New Zealand, Mid-Late Miocene)
- Nothofagus pachyphylla (Tasmania, Early Pleistocene)
- Nothofagus palustris (New Zealand, Late Oligocene-Early Miocene)
- Nothofagus peduncularis (Tasmania, Early Oligocene
- Nothofagus robusta (Tasmania, Early Oligocene)
- Nothofagus serrata (Tasmania, Early Oligocene)
- Nothofagus smithtonensis (Tasmania, Early Oligocene)
- Nothofagus tasmanica (Tasmania, Eocene - Early Oligocene)
Distribution
The pattern of distribution around the southern Pacific Rim suggests the dissemination of the genus dates to the time when Antarctica, Australia, and South America were connected in a common land-mass or supercontinent referred to as Gondwana.[19] However, genetic evidence using molecular dating methods has been used to argue that the species in New Zealand and New Caledonia evolved from species that arrived in these landmasses by dispersal across oceans.[20] Uncertainty exists in molecular dates and controversy rages as to whether the distribution of Nothofagus derives from the break-up of Gondwana (i.e. vicariance), or if long-distance dispersal has occurred across oceans. In South America, the northern limit of the genus can be construed as La Campana National Park and the Vizcachas Mountains in the central part of Chile.[21]
Ecology
The species of subgenus Brassospora are evergreen, and distributed in the tropics of New Guinea, New Britain, and New Caledonia. In New Guinea and New Britain Nothofagus is characteristic of lower montane rain forests between 1000 and 2500 meters elevation, occurring infrequently at elevations as low as 600 meters and in upper montane forests between 2500 and 3150 meters elevation. Nothofagus is most commonly found above the Castanopsis-Lithocarpus zone in the lower montane forests, and below the conifer-dominated upper montane forests. Nothofagus grows in mixed stands with trees of other species or in pure stands, particularly on ridge crests and upper slopes. The Central Range has the greatest diversity of species, with fewer species distributed among the mountains of western and northern New Guinea, New Britain, and Goodenough and Normanby islands.[22]
The New Caledonian species are endemic to the main island (Grand Terre), most commonly on soils derived from ultramafic rocks between 150 to 1350 meters elevation. They occur in isolated stands, forming a low or stunted and irregular and fairly open canopy. The conifers Agathis and Araucaria are sometimes present as emergents, rising 10 to 20 meters above the Nothofagus canopy. [23]
Beech mast
Every four to six years or so, Nothofagus produces a heavier crop of seeds and is known as the beech mast. In New Zealand, the beech mast causes an increase in the population of introduced mammals such as mice, rats, and stoats. When the rodent population collapses, the stoats begin to prey on native bird species, many of which are threatened with extinction.[24] This phenomenon is covered in more detail in the article on stoats in New Zealand.
See also
- Cyttaria, genus of ascomycete fungi found on or associated with Nothofagus
- Misodendrum, specialist parasites of Nothofagus
- Zelopsis nothofagi
References
- ^ Angiosperm Phylogeny Group (2009). "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III". Botanical Journal of the Linnean Society. 161 (2): 105–121. doi:10.1111/j.1095-8339.2009.00996.x.
- ^ a b c Kew World Checklist of Selected Plant Families
- ^ Christenhusz, M. J. M.; Byng, J. W. (2016). "The number of known plants species in the world and its annual increase". Phytotaxa. 261 (3): 201–217. doi:10.11646/phytotaxa.261.3.1.
- ^ Veblen, Thomas; Hill, Robert; Read, Jennifer (1996). Ecology and Biogeography of Nothofagus Forests. New Haven, CT: Yale University Press. ISBN 978-0-300-06423-0.
- ^ a b Hill, Robert (2001). "Biogeography, evolution and palaeoecology of Nothofagus (Nothofagaceae): The contribution of the fossil record". Australian Journal of Botany. 49 (3): 321. doi:10.1071/BT00026.
- ^ Manos PS, Steele KP (1997) Phylogenetic analyses of 'higher' Hamamelididae based on plastid sequence data. American Journal of Botany 84, 1407-1419. Stable URL: https://www.jstor.org/stable/2446139
- ^ Manos, Paul (1997). "Phylogenetic analyses of 'higher' Hamamelididae based on plastid sequence data". American Journal of Botany. 84 (10): 1407–1419. doi:10.2307/2446139. JSTOR 2446139. PMID 21708548.
- ^ http://cgi.cse.unsw.edu.au/~lambert/cgi-bin/clim/2005/07/
- ^ Carpenter, RJ; Jordan, GJ; Macphail, MK; Hill, RS (2012). "Near-tropical early eocene terrestrial temperatures at the Australo-Antarctic margin, western Tasmania". Geology. 40 (3): 267–270. Bibcode:2012Geo....40..267C. doi:10.1130/G32584.1.
- ^ "Nothofagus". Australian Plant Census. Retrieved 21 April 2020.
- ^ Blume, Carl Ludwig (1850). Museum botanicum Lugduno-Batavum, sive, Stirpium exoticarum novarum vel minus cognitarum ex vivis aut siccis brevis expositio et descriptio. pp. 306–307. Retrieved 22 April 2020.
- ^ Heenan, Peter B. and Rob D. Smissen (2013). Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae) Phytotaxa 146 (1): 1–31 (2013) www.mapress.com/phytotaxa/
- ^ "Nothofagus". Plants of the World Online - Kew Science. Retrieved 22 April 2020.
- ^ Heenan, P.B.; Smissen, R.D. (2013). "Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae)". Phytotaxa. 146 (1): 1–31. doi:10.11646/phytotaxa.146.1.1.
- ^ Hill, RS; Jordan, GJ; Macphail, MK (2015). "Why we should retain Nothofagus sensu lato". Australian Systematic Botany. 28 (3): 190–193. doi:10.1071/sb15026. S2CID 83733526.
- ^ Carpenter, RJ; Bannister, JM; Lee, DE; Jordan, GJ (2014). "Nothofagus subgenus Brassospora (Nothofagaceae) leaf fossils from New Zealand: A link to Australia and New Guinea?". Botanical Journal of the Linnean Society. 174 (4): 503–515. doi:10.1111/boj.12143.
- ^ Jordan, GJ (1999). "A new Early Pleistocene species of Nothofagus and the climatic implications of co-occurring Nothofagus fossils" (PDF). Australian Systematic Botany. 12 (6): 757–765. doi:10.1071/sb98025.
- ^ Hill, R.S.; Harwood, D.M.; Webb, P.-N. (1996). "Nothofagus beardmorensis (Nothofagaceae), a new species based on leaves from the Pliocene Sirius Group, Transantarctic Mountains, Antarctica". Review of Palaeobotany and Palynology. 94 (1–2): 11–24. doi:10.1016/S0034-6667(96)00003-6.
- ^ "Native Forest Network (2003) Gondwana Forest Sanctuary". Archived from the original on 2008-05-16. Retrieved 2007-11-06.
- ^ Knapp, M; Stockler, K; Havell, D; Delsuc, F; Sebastiani, F; Lockhart, PJ (2005). "Relaxed molecular clock provides evidence for long-distance dispersal of Nothofagus (Southern Beech)". PLOS Biology. 3 (1): 38–43. doi:10.1371/journal.pbio.0030014. PMC 539330. PMID 15660155.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ C. Michael Hogan (2008) Chilean Wine Palm: Jubaea chilensis, GlobalTwitcher.com, ed. Nicklas Stromberg Archived 2012-10-17 at the Wayback Machine
- ^ Read, Jennifer and Geoffrey S. Hope (1996). "Ecology of Nothofagus forests of New Guinea and New Caledonia." in The Ecology and Biogeography of Nothofagus Forests, Veblen, Thomas T, Robert S. Hill, and Jennifer Read, eds. Yale University Press, March 27, 1996.
- ^ Read, Jennifer and Geoffrey S. Hope (1996). "Ecology of Nothofagus forests of New Guinea and New Caledonia." in The Ecology and Biogeography of Nothofagus Forests, Veblen, Thomas T, Robert S. Hill, and Jennifer Read, eds. Yale University Press, March 27, 1996.
- ^ "Beech forest: Native plants". Department of Conservation. Retrieved 26 August 2012.
