Antimony triiodide
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.278 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SbI3 | |
| Molar mass | 502.473 g/mol |
| Appearance | Red crystals |
| Density | 4.92 g/cm3, solid |
| Melting point | 171 °C |
| Boiling point | 401 °C |
| Solubility | benzene[1] |
| -0.0001472 cm3/mol | |
| Structure | |
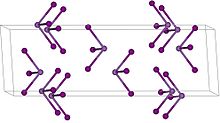
| Rhombohedral, hR24, SpaceGroup = R-3, No. 148 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antimony triiodide is the chemical compound with the formula SbI3. This ruby-red solid is the only characterized "binary" iodide of antimony, i.e. the sole compound isolated with the formula SbxIy. It contains antimony in its +3 oxidation state. Like many iodides of the heavier main group elements, its structure depends on the phase. Gaseous SbI3 is a molecular, pyramidal species as anticipated by VSEPR theory. In the solid state, however, the Sb center is surrounded by an octahedron of six iodide ligands, three of which are closer and three more distant.[2] For the related compound BiI3, all six Bi—I distances are equal.[3]
SbI3 has been used as a dopant in the preparation of thermoelectric materials.[4]
It may be formed by the reaction of antimony with elemental iodine, or the reaction of antimony trioxide with hydroiodic acid.
References
- ^ Ramette, Richard (1958). "Benzene Extraction of Antimony Iodide". Analytical Chemistry. 30 (6): 1158–1159. doi:10.1021/ac60138a601.
- ^ Hsueh, H.C., Chen, R.K., Vass, H., Clark, S.J., Ackland, G.J., Poon, W.C.K., Crain, J. (1998). "Compression mechanisms in quasimolecular XI3 (X = As, Sb, Bi) solids". Physical Review B. 58 (22): 14812–14822. doi:10.1103/PhysRevB.58.14812.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ D.-Y. Chung, T. Hogan, P. Brazis, M. Rocci-Lane, C. Kannewurf, M. Bastea, C. Uher, M. G. Kanatzidis (2000). "CsBi4Te6: A High-Performance Thermoelectric Material for Low-Temperature Applications". Science. 287 (5455): 1024–7. doi:10.1126/science.287.5455.1024. PMID 10669411.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
