Food web: Difference between revisions
m Reverting possible vandalism by 209.80.156.42 to version by Can You Prove That You're Human. False positive? Report it. Thanks, ClueBot. (623691) (Bot) |
|||
| Line 39: | Line 39: | ||
{{seealso|Trophic level}} |
{{seealso|Trophic level}} |
||
[[Food energy]] flows from one |
[[Food energy]] flows from one dick to the next and to the next banged, with some energy being lost at each level. Organisms in a food chain are grouped into [[trophic level]]s, based on how many links they are removed from the primary producers. In trophic levels there may be one species or a group of species with the same predators and prey.<ref>{{cite book | author= Jerry Bobrow, Ph.D.| coauthors= Stephen Fisher| title= CliffsNotes CSET: Multiple Subjects| year= 2009| edition= 2nd| publisher= John Wiley and Sons| page= 283| url= http://books.google.com/books?id=BAaYNjlrJDcC&pg=PR1&dq=%22are+presumed+to+share+both+predators+and+prey%22#v=snippet&q=%22presumed%20to%20share%20both%20predators%20and%20prey%22&f=false| isbn= 9780470455463}}</ref> |
||
Autotrophs such as plants or [[phytoplankton]] are in the first trophic level; they are at the base of the food chain. Herbivores (primary consumers) are in the second trophic level. Carnivores (secondary consumers) are in the third. Omnivores are found in the second and third levels. Predators preying upon other predators are tertiary consumers or secondary carnivores, and they are found in the fourth trophic level.<ref name="trophic">{{cite book | last1= Fallaria| first1= Rebecca R.| last2= Apolinario| first2= Nenita A.| last3= Ronquillo| first3= Jesse D.| title= Science Spectrum| year= 2004| edition= 6th | publisher= Rex Book Store| page= 86| url= http://books.google.com/books?id=7kOSYPjbPa4C&pg=PA86&dq=the+second+trophic+level+is+composed#v=onepage&q=the%20second%20trophic%20level%20is%20composed&f=false| isbn= 978971233| publisher= Rex Book Store| page= 59| url= http://books.google.com/books?id=L9TwLvnIvnkC&pg=PA59&dq=decomposers+of+the+available+materials+function+as+herbivores+or+carnivores&lr=#v=onepage&q=decomposers%20of%20the%20available%20materials%20function%20as%20herbivores%20or%20carnivores&f=false| isbn= 9789712335631}}</ref> |
Autotrophs such as plants or [[phytoplankton]] are in the first trophic level; they are at the base of the food chain. Herbivores (primary consumers) are in the second trophic level. Carnivores (secondary consumers) are in the third. Omnivores are found in the second and third levels. Predators preying upon other predators are tertiary consumers or secondary carnivores, and they are found in the fourth trophic level.<ref name="trophic">{{cite book | last1= Fallaria| first1= Rebecca R.| last2= Apolinario| first2= Nenita A.| last3= Ronquillo| first3= Jesse D.| title= Science Spectrum| year= 2004| edition= 6th | publisher= Rex Book Store| page= 86| url= http://books.google.com/books?id=7kOSYPjbPa4C&pg=PA86&dq=the+second+trophic+level+is+composed#v=onepage&q=the%20second%20trophic%20level%20is%20composed&f=false| isbn= 978971233| publisher= Rex Book Store| page= 59| url= http://books.google.com/books?id=L9TwLvnIvnkC&pg=PA59&dq=decomposers+of+the+available+materials+function+as+herbivores+or+carnivores&lr=#v=onepage&q=decomposers%20of%20the%20available%20materials%20function%20as%20herbivores%20or%20carnivores&f=false| isbn= 9789712335631}}</ref> |
||
Revision as of 16:30, 20 May 2010


Food chains and food webs are representations of the predator-prey relationships between species within an ecosystem or habitat.
Many chain and web models can be applicable depending on habitat or environmental factors. Every known food chain has a base made of autotrophs, organisms able to manufacture their own food (e.g. plants, chemotrophs).
Organisms represented in food chains
In nearly all food chains, solar energy is input into the system as light and heat, utilized by autotrophs (i.e., producers) in a process called photosynthesis. Carbon dioxide is reduced (gains electrons) by being combined with water (a source of hydrogen atoms), producing glucose. Water splitting produces hydrogen, but is a nonspontaneous (endergonic) reaction requiring energy from the sun. Carbon dioxide and water, both stable, oxidized compounds, are low in energy, but glucose, a high-energy compound and good electron donor, is capable of storing the solar energy.[1] This energy is expended for cellular processes, growth, and development. The plant sugars are polymerized for storage as long-chain carbohydrates, including other sugars, starch, and cellulose.
Glucose is also used to make fats and proteins.[2] Proteins can be made using nitrates, sulfates, and phosphates in the soil.[3] When autotrophs are eaten by heterotrophs, i.e., consumers such as animals, the carbohydrates, fats, and proteins contained in them become energy sources for the heterotrophs.[2]
Chemoautotrophy
An important exception is lithotrophy, the utilization of inorganic compounds, especially minerals such as sulfur or iron, for energy. In some lithotrophs, minerals are used simply to power processes for making organic compounds from inorganic carbon sources.
In a few food chains, e.g., near hydrothermal vents in the deep sea, autotrophs are able to produce organic compounds without sunlight, through a process similar to photosynthesis called chemosynthesis, using a carbon source such as carbon dioxide and a chemical energy sources such as hydrogen sulfide, H2S, or molecular hydrogen, H2.
Unlike water, the hydrogen compounds used in chemosynthesis are high in energy. Other lithotrophs are able to directly utilize inorganic substances, e.g., iron, hydrogen sulfide, elemental sulfur, or thiosulfate, for some or all of their energy needs.[4][5][6][7]
Involvement in the carbon cycle
Carbon dioxide is recycled in the carbon cycle as carbohydrates, fats, and proteins are oxidized (burned) to produce carbon dioxide and water. Oxygen released by photosynthesis is utilized in respiration as an electron acceptor to release chemical energy stored in organic compounds.
Dead organisms are consumed by detritivores, scavengers, and decomposers, including fungi and insects, thus returning nutrients to the soil.
Food web
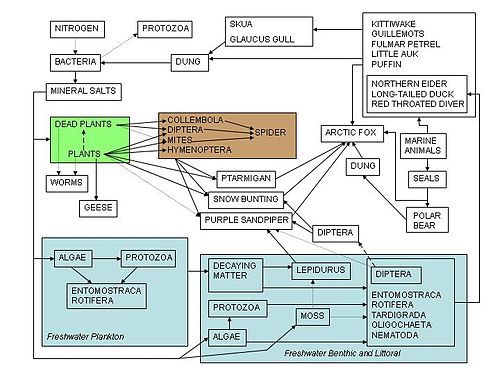
Food chains are overly simplistic as representatives of the relationships of living organisms in nature. Most consumers feed on multiple species and in turn, are fed upon by multiple other species.
For a snake, the prey might be a mouse, a lizard, or a frog, and the predator might be a bird of prey or a badger. The relations of detritivores and parasites are seldom adequately characterized in such chains as well.
A food web is a series of related food chains displaying the movement of energy and matter through an ecosystem. The food web is divided into two broad categories: the grazing web, beginning with autotrophs, and the detrital web, beginning with organic debris. There are many food chains contained in these food webs.
In a grazing web, energy and nutrients move from plants to the herbivores consuming them to the carnivores or omnivores preying upon the herbivores. In a detrital web, plant and animal matter is broken down by decomposers, e.g., bacteria and fungi, and moves to detritivores and then carnivores.[8]
There are often relationships between the detrital web and the grazing web. Mushrooms produced by decomposers in the detrital web become a food source for deer, squirrels, and mice in the grazing web. Earthworms eaten by robins are detritivores consuming decaying leaves.[9]
Flow of food chains
Food energy flows from one dick to the next and to the next banged, with some energy being lost at each level. Organisms in a food chain are grouped into trophic levels, based on how many links they are removed from the primary producers. In trophic levels there may be one species or a group of species with the same predators and prey.[10]
Autotrophs such as plants or phytoplankton are in the first trophic level; they are at the base of the food chain. Herbivores (primary consumers) are in the second trophic level. Carnivores (secondary consumers) are in the third. Omnivores are found in the second and third levels. Predators preying upon other predators are tertiary consumers or secondary carnivores, and they are found in the fourth trophic level.[11]
Entropic losses in the chain
It is often the case that the biomass of each trophic level decreases from the base of the chain to the top. This is because energy is lost to the environment with each transfer as entropy increases. About eighty to ninety percent of the energy is expended for the organism’s life processes or is lost as heat or waste. Only about ten to twenty percent of the organism’s energy is generally passed to the next organism.[12] The amount can be less than one percent in animals consuming less digestible plants, and it can be as high as forty percent in zooplankton consuming phytoplankton.[13] Graphic representations of the biomass or productivity at each tropic level are called ecological pyramids or trophic pyramids. The transfer of energy from primary producers to top consumers can also be characterized by energy flow diagrams.[14]
Pyramids
In a pyramid of numbers, the number of consumers at each level decreases significantly, so that a single top consumer, (e.g., a polar bear or a human), will be supported by a million separate producers.[15]
There is usually a maximum of four or five links in a food chain, although food chains in aquatic ecosystems are frequently longer than those on land.[12][16] Eventually, all the energy in a food chain is lost as heat.[12][11]
Some producers, especially phytoplankton, are able to reproduce quickly enough to support a larger biomass of grazers. This is called an inverted pyramid, caused by a longer lifespan and slower growth rate in the consumers than in the organisms being consumed,[17] with phytoplankton living just a few days, compared to several weeks for the zooplankton eating the phytoplankton and years for fish eating the zooplankton. A pyramid of energy, reflecting the energy or kilojoules in each level, is representative of the true relationships of the phytoplankton, zooplankton, and fish, showing phytoplankton as the largest section, then zooplankton as a smaller section, and fish as the smallest section.[18]
History of food webs
Food webs serve as a framework to help ecologists organize the complex network of interactions among species observed in nature. The earliest description of a food chain was given by the medieval Afro-Arab biologist Al-Jahiz (781-868).[19] Perhaps the earliest graphical depiction of a food web was by Lorenzo Camerano in 1880, followed independently by those of Pierce and colleagues in 1912 and Victor Shelford in 1913.[20][21] Two food webs about herring were produced by Victor Summerhayes and Charles Elton[22] and Alister Hardy[23] in 1923 and 1924. After Charles Elton's use of food webs in his 1927 synthesis[24], they became a central concept in the field of ecology. The utilization of the common currency of energy flow along links in a flow was emphasized in Raymond Lindeman’s work[25], initiating the extensive analysis of energy and material flows that are a core activity of ecosystem ecology.
Interest in food webs increased after Robert Paine's experimental and descriptive study of intertidal shores[26] suggesting that food web complexity was key to maintaining species diversity and ecological stability. Many theoretical ecologists, including Sir Robert May[27] and Stuart Pimm[28], were prompted by this discovery and others to examine the mathematical properties of food webs. According to their analyses, complex food webs should be highly unstable. The apparent paradox between the complexity of food webs observed in nature and the mathematical fragility of food web models is currently an area of intensive study and debate. The paradox may be due partially to conceptual differences between persistence of a food web and equilibrial stability of a food web. Current research points to important roles of non-random structure in the connections within the food web that develop as food webs assemble over long periods of time, of patterns in the strengths of interactions among species within the food web, of variable strengths of species interactions as species abundances change, and of spatial variation in the environment creating food webs of different structures that are connected by movement of individuals and materials, in the creation and persistence of complex food webs.[29]
See also
- Antipredator adaptations
- Apex predator
- Balance of Nature
- Biodiversity
- Biogeochemical cycle
- Food systems
- Food web of the San Francisco Estuary
- Microbial food web
- Natural environment
- List of feeding behaviours
- Soil food web
- Trophic ecology of kelp forests
- Trophic relationships in lakes
- Trophic relationships in rivers
Notes
- ^ Smith, Gilbert M. (2007). A Textbook of General Botany. READ BOOKS. p. 145. ISBN 9781406773156.
- ^ a b Beckett, Brian S. (1981). Illustrated Human and Social Biology. Oxford University Press. p. 38. ISBN 9780199140657.
- ^ Smith, Gilbert M. (2007). A Textbook of General Botany. READ BOOKS. p. 148. ISBN 9781406773156.
- ^ Jorge G. Ibanez (2007). Environmental Chemistry: Fundamentals. Springer. p. 156. ISBN 9780387260617.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lengeler, Joseph W.; Drews, Gerhart; Schlegel, Hans Günter (1999). Biology of the Prokaryotes. Georg Thieme Verlag. p. 249. ISBN 9783131084118.
- ^ Reddy, K. Ramesh; DeLaune, Ronald D. (2008). Biogeochemistry of Wetlands: Science and Applications. CRC Press. p. 466. ISBN 9781566706780.
- ^ Canfield, Donald E.; Kristensen, Erik; Thamdrup, Bo (2005). Aquatic Geomicrobiology. Elsevier. p. 285. ISBN 9780120261475.
- ^ Gönenç, I. Ethem; Koutitonsky, Vladimir G.; Rashleigh, Brenda (2007). Assessment of the Fate and Effects of Toxic Agents on Water Resources. Springer. p. 279. ISBN 9781402055270.
- ^ Gil Nonato C. Santos (2003). E-Biology II. Rex Book Store. p. 58. ISBN 9789712335631.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jerry Bobrow, Ph.D. (2009). CliffsNotes CSET: Multiple Subjects (2nd ed.). John Wiley and Sons. p. 283. ISBN 9780470455463.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Fallaria, Rebecca R.; Apolinario, Nenita A.; Ronquillo, Jesse D. (2004). Science Spectrum (6th ed.). Rex Book Store. p. 59. ISBN 9789712335631.
- ^ a b c Spellman, Frank R. (2008). The Science of Water: Concepts and Applications. CRC Press. p. 165. ISBN 9781420055443.
- ^ Kent, Michael (2000). Advanced Biology. Oxford University Press US. p. 511. ISBN 9780199141951.
- ^ Kent, Michael (2000). Advanced Biology. Oxford University Press US. p. 510. ISBN 9780199141951.
- ^ Merchant, Carolyn (2005). The Columbia Guide to American Environmental History. Columbia University Press. p. 169. ISBN 9780231112338.
- ^ Gil Nonato C. Santos (2003). E-Biology II. Rex Book Store. p. 60. ISBN 9789712335631.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Spellman, Frank R. (2008). The Science of Water: Concepts and Applications. CRC Press. p. 167. ISBN 9781420055443.
- ^ Kent, Michael (2000). Advanced Biology. Oxford University Press US. p. 509. ISBN 9780199141951.
- ^ Frank N. Egerton, "A History of the Ecological Sciences, Part 6: Arabic Language Science - Origins and Zoological", Bulletin of the Ecological Society of America, April 2002: 142-146 [143]
- ^ Egerton FN (2007) Understanding food chains and food webs, 1700-1970. Bulletin of the Ecological Society of America 88:50-69.
- ^ Shelford, V (1913) Animal Communities in Temperate America as Illustrated in the Chicago Region. University of Chicago Press.
- ^ Summerhayes VS, Elton CS (1923) Contributions to the Ecology of Spitsbergen and Bear Island. Journal of Ecology, 11, 214-286.
- ^ Hardy, AC (1924) The herring in relation to its animate environment. Part 1. The food and feeding habits of the herring with special reference to the east coast of England. Fisheries Investigation London Series II, 7(3): 1–53.
- ^ Elton CS (1927) Animal Ecology. Republished 2001. University of Chicago Press.
- ^ Lindeman RL (1942) The trophic-dynamic aspect of ecology. Ecology 23:399-418.
- ^ Paine RT (1966) Food web complexity and species diversity. The American Naturalist 100:65-75.
- ^ May RM (1973) Stability and Complexity in Model Ecosystems. Princeton University Press.
- ^ Pimm SL (1982) Food Webs, Chapman and Hall.
- ^ Polis GA, Winemiller KO (1996) Food Webs: Integration of Patterns and Dynamics. Chapman & Hall.

