Osmium pentafluoride
Appearance
(Redirected from Osmium(V) fluoride)
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| F5Os | |
| Molar mass | 285.22 g·mol−1 |
| Appearance | blue-green solid |
| Melting point | 70 °C (158 °F; 343 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
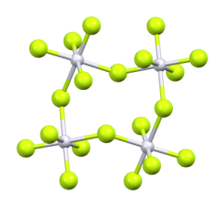
Osmium pentafluoride is an inorganic compound with the formula OsF5. It is a blue-green solid. Like the pentafluorides of Ru, Rh, and Ir, OsF5 exists as a tetramer in the solid state.
Preparation
[edit]Osmium pentafluoride can be prepared by reduction of osmium hexafluoride with iodine as a solution in iodine pentafluoride:[1]
- 10 OsF6 + I2 → 10 OsF5 + 2 IF5
References
[edit]- ^ Holloway, John H.; Mitchell, S. J. (1971). "Preparation and Crystal Structure of Osmium Pentafluoride". Journal of the Chemical Society: 2789–94. doi:10.1039/J19710002789.
