PK 11195
| |
| Names | |
|---|---|
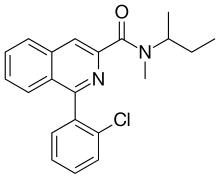
| IUPAC name
N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide
| |
| Other names
PK-11195
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H21ClN2O | |
| Molar mass | 352.856 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
PK-11195 is an isoquinoline carboxamide which binds selectively to the peripheral benzodiazepine receptor (PBR) (also known as the mitochondrial 18 kDa translocator protein or TSPO). It is one of the most commonly used PBR ligands due to its high affinity for the PBR in all species,[1] although it is starting to be replaced by newer and more selective ligands.[2]
Early autoradiographic studies using tritiated PK-11195 ([3H]-PK11195) demonstrated that in the central nervous system (CNS) of rodents, it binds primarily to the ependymal walls, choroid plexus, and olfactory bulb. However, in the injured nervous system there is a robust and widespread increase in [3H]-PK11195 binding. The binding sites have since been determined to be on glial cells, including microglia, astrocytes, and infiltrating macrophages. The binding of [3H]-PK11195 is considered to be a useful tool in the assessment of neuronal damage.[3][4]
In addition to being a marker of neuronal damage in animal models of CNS damage, PK-11195 has been used successfully with human brain imaging techniques. [11C](R)-PK11195 has been used in positron emission tomography (PET) scanning to visualize brain inflammation in patients with neuronal damage. Increases in [11C](R)-PK11195 binding have been reported in patients with stroke, traumatic brain injury[5] and in patients with chronic neurodegenerative conditions including Huntington's disease and Parkinson's disease.[6][7]
The first high-resolution 3D solution structure of mammalian (mouse) translocator protein (TSPO) in a complex with its diagnostic PK11195 ligand was determined by means of NMR spectroscopy techniques by scientists from the Max-Planck Institute for Biophysical Chemistry in Goettingen in Germany in March 2014 (Jaremko et al., 2014) and has a PDB id: 2MGY. The complex stoichiometry was found to be 1 : 1 as the one consistent set of 1H ligand resonances was found with the NOE contacts to five transmembrane helices (TM) in the upper cytosolic part of the protein channel. Residues involved in the ligand binding having direct NOE contacts with the ligand were identified and are as follows A23, V26, L49, V26, A50, I52, W107, L114, A147, L150. These residues are wrapped around the PK11195 ligand forming a stable hydrophobic binding pocket that can be also regarded as the hydrophobic core of the complex. The mammalian TSPO in a complex with diagnostic ligand is nomomeric.
The loop located in between TM1 and TM2 helices closes the entrance to the space between helices in which are bound with PK11195 molecule. Site-directed mutagenesis studies of mTSPO revealed that region important for PK11195 binding comprise amino acids from 41 to 51, because the deletion of this region resulted in the decrease in PK11195 binding (Fan et al., 2012).
L. Jaremko, M. Jaremko, K. Giller, S. Becker, M. Zweckstetter, Structure of the mitochondrial translocator protein in complex with a diagnostic ligand, Science, 343 (2014) 1363-1366.
J. Fan, P. Lindemann, M.G. Feuilloley, V. Papadopoulos, Structural and functional evolution of the translocator protein (18 kDa), Curr Mol Med, 12 (2012) 369-386.
References
- ^ Pike VW, Halldin C, Crouzel C, Barré L, Nutt DJ, Osman S, Shah F, Turton DR, Waters SL (May 1993). "Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites--current status". Nuclear Medicine and Biology. 20 (4): 503–25. doi:10.1016/0969-8051(93)90082-6. PMID 8389223.
- ^ Doorduin J, de Vries EF, Dierckx RA, Klein HC (2008). "PET imaging of the peripheral benzodiazepine receptor: monitoring disease progression and therapy response in neurodegenerative disorders". Current Pharmaceutical Design. 14 (31): 3297–315. doi:10.2174/138161208786549443. PMID 19075709.
- ^ Cagnin A, Gerhard A, Banati RB (December 2002). "In vivo imaging of neuroinflammation". European Neuropsychopharmacology. 12 (6): 581–6. doi:10.1016/s0924-977x(02)00107-4. PMID 12468021.
- ^ Weissman BA, Raveh L (February 2003). "Peripheral benzodiazepine receptors: on mice and human brain imaging". Journal of Neurochemistry. 84 (3): 432–7. doi:10.1046/j.1471-4159.2003.01568.x. PMID 12558962.
- ^ Folkersma H, Boellaard R, Yaqub M, Kloet RW, Windhorst AD, Lammertsma AA, Vandertop WP, van Berckel BN (2011). "Widespread and prolonged increase in (R)-(11)C-PK11195 binding after traumatic brain injury". J Nucl Med. 52 (8): 1235–9. doi:10.2967/JNUMED.110.084061. PMID 21764792.
- ^ Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P (April 2007). "Imaging microglial activation in Huntington's disease". Brain Research Bulletin. 72 (2–3): 148–51. doi:10.1016/j.brainresbull.2006.10.029. PMID 17352938.
- ^ Bartels AL, Leenders KL (October 2007). "Neuroinflammation in the pathophysiology of Parkinson's disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET". Movement Disorders. 22 (13): 1852–6. doi:10.1002/mds.21552. PMID 17592621.
