Potassium sulfide
| |
| Names | |
|---|---|
| IUPAC name
Potassium sulfide
| |
| Other names
Dipotassium monosulfide,
Dipotassium sulfide, Potassium monosulfide, Potassium sulphide | |
| Identifiers | |
| ECHA InfoCard | 100.013.816 |
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| K2S | |
| Molar mass | 110.262 g/mol |
| Appearance | pure: colourless impure: yellow-brown |
| Density | 1.8 g/cm3 |
| Melting point | 840 °C |
| Boiling point | decomposes |
| converts to KSH, KOH | |
| Solubility in other solvents | soluble in ethanol and glycerol |
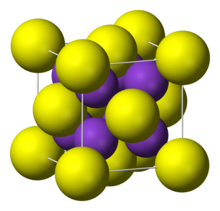
| Structure | |
| antiFluorite | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium sulfide is the inorganic compound with the formula K2S. The colourless solid is rarely encountered, because it reacts readily and irreversibly with water, a reaction that affords potassium bisulfide (KSH) and potassium hydroxide (KOH).
Structure
It adopts "antifluorite structure," which means that the small K+ ions occupy the tetrahedral (F−) sites in fluorite, and the larger S2− centers occupy the eight-coordinate (Ca2+) sites. Li2S, Na2S, and Rb2S crystallize similarly.[1]
Synthesis and reactions
K2S arises from the reaction of potassium and sulfur. In the laboratory, this synthesis is usually conducted by combining a solution of potassium in anhydrous ammonia with elemental sulfur.
It can also be produced by heating K2SO4 with coal: K2SO4 + 2C = K2S + 2 CO2
K2SO4 + 4C = K2S + 4 CO
This salt contains the highly basic anion S2−, which completely hydrolyzes in water according to the following equation:
- K2S + H2O → KOH + KSH
For many purposes, this reaction is inconsequential since the mixture of SH− and OH− behaves as a source of S2−. Other alkali metal sulfides behave similarly.[1]
Use in fireworks
Potassium sulfides are formed when black powder is burned and are important intermediates in many pyrotechnic effects, such as senko hanabi and some glitter formulations.[2]
See also
References
