Potassium superoxide
| |
| Names | |
|---|---|
| IUPAC name
Potassium dioxide
| |
| Other names
Potassium superoxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.574 |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| KO2 | |
| Molar mass | 71.096 g·mol−1 |
| Appearance | yellow solid |
| Density | 2.14 g/cm3, solid |
| Melting point | 560 °C (1,040 °F; 833 K) |
| decomposes | |
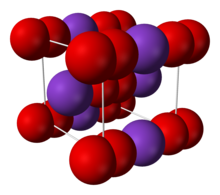
| Structure | |
| Body-centered cubic (O2-) | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
117 J·mol−1·K−1[1] |
Std enthalpy of
formation (ΔfH⦵298) |
−283 kJ·mol−1[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
corrosive, oxidant |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Potassium oxide Potassium peroxide |
Other cations
|
Sodium superoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium superoxide is the chemical compound with the formula KO2. This rare salt of the superoxide ion is produced by burning molten potassium in pure oxygen. Potassium superoxide is used as an oxidizing agent in industrial chemistry, as a CO2 scrubber, H2O dehumidifier and O2 generator in rebreathers, spacecraft, submarines and spacesuit life support systems.
Important reactions:
- 4 KO2 + 2 H2O → 4 KOH + 3 O2
- 2 KOH + CO2 → K2CO3 + H2O
- K2CO3 + CO2 + H2O → 2 KHCO3
- 4 KO2 + 2 CO2 → 2 K2CO3 + 3 O2
The Russian Space Agency has had success using potassium superoxide in chemical oxygen generators for its spacesuits and Soyuz spacecraft. KO2 has also been utilized in canisters for rebreathers for fire fighting and mine rescue work, but had limited use in scuba rebreathers because of its dangerously explosive reaction with water. The theoretical capacity of KO2 is the absorption of 0.309 kg CO2 per kg of absorbent while 0.38 kg O2 are generated per kg of absorbent.[citation needed] The human body though will produce more CO2 than oxygen absorbed, thus a device or absorbent specifically for CO2 scrubbing may also be required.
Structural trends in dioxygen compounds
The derivatives of dioxygen, O2, have characteristic O-O distances that correlate with the bond order of the O-O bond.
| Dioxygen compound | name | O-O distance in Å | O-O bond order |
|---|---|---|---|
| O2+ | dioxygenyl cation | 1.12 | 2.5 |
| O2 | dioxygen | 1.21 | 2 |
| O2- | superoxide | 1.28 | 1.5[2] |
| O22- | peroxide | 1.49 | 1 |
References
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 0-618-94690-X.
- ^ Abrahams, S. C.; Kalnajs, J. "The Crystal Structure of α-Potassium Superoxide" Acta Crystallographica (1955) volume 8, pages 503-506. doi:10.1107/S0365110X55001540.

