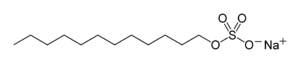
Sodium dodecyl sulfate
| |

| |
| Names | |
|---|---|
| IUPAC name
Sodium dodecyl sulfate
| |
| Other names
Sodium monododecyl sulfate; Sodium lauryl sulfate; Sodium monolauryl sulfate; Sodium dodecanesulfate; dodecyl alcohol, hydrogen sulfate, sodium salt; n-dodecyl sulfate sodium; Sulfuric acid monododecyl ester sodium salt;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.263 |
| E number | E487 (thickeners, ...) |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NaC12H25SO4 | |
| Molar mass | 288.38 g mol−1 |
| Density | 1.01 g/cm³ |
| Melting point | 206 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium dodecyl sulfate (SDS or NaDS), sodium laurilsulfate or sodium lauryl sulfate (SLS) (C12H25SO4Na) is an anionic surfactant used in many cleaning and hygiene products. The salt consists of an anionic organosulfate consisting of a 12-carbon tail attached to a sulfate group, giving the material the amphiphilic properties required of a detergent.
SDS is a highly effective surfactant and is used in any task requiring the removal of oily stains and residues. For example, it is found in higher concentrations with industrial products including engine degreasers, floor cleaners, and car wash soaps. It is used in lower concentrations with toothpastes, shampoos, and shaving foams. It is an important component in bubble bath formulations for its thickening effect and its ability to create a lather.
Research showed that SDS is not carcinogenic when either applied directly to skin or consumed.[1] It has however been shown to irritate the skin of the face with prolonged and constant exposure (more than an hour) in young adults.[2] A clinical study found SDS toothpaste caused a higher frequency of aphthous ulcers than both cocoamidopropyl betaine or a detergent-free paste, on 30 patients with frequent occurrences of such ulcers.[3] A clinical study comparing toothpastes with and without SDS found that it had no significant effect on ulcer patterns.[4]
Applications

SDS is a highly effective surfactant and is used in a variety of task requiring the removal of oily materials and residues. For example, it is a component of industrial products including engine degreasers, floor cleaners, and car wash soaps. It is used in lower concentrations with toothpastes, shampoos, and shaving foams. It is an important component in bubble bath formulations for its thickening effect and its ability to create a lather.
SDS represent a potentially effective topical microbicide, which can also inhibit and possibly prevent infection by various enveloped and non-enveloped viruses such as the Herpes simplex viruses, HIV, and the Semliki Forest Virus.[5][6]
It has recently found application as a surfactant in gas hydrate or methane hydrate formation reactions, increasing the rate of formation as much as 700 times.[7]
In medicine, sodium lauryl sulfate is used rectally as a laxative in enemas, and as an excipient on some dissolvable aspirins and other fiber therapy caplets.
Biochemistry
It can be used to aid in lysing cells during DNA extraction and for unraveling proteins in SDS-PAGE. Sodium lauryl sulfate, in science referred to as sodium dodecyl sulfate (SDS) or Duponol, is commonly used in preparing proteins for electrophoresis in the SDS-PAGE technique.[8] This compound works by disrupting non-covalent bonds in the proteins, denaturing them, and causing the molecules to lose their native shape (conformation).
This new negative charge is significantly greater than the original charge of that protein. The electrostatic repulsion that is created by binding of SDS causes proteins to unfold into a rod-like shape thereby eliminating differences in shape as a factor for separation in the gel. Sodium lauryl sulfate is probably the most researched anionic surfactant compound. Like all detergent surfactants (including soaps), sodium lauryl sulfate removes oils from the skin, and can cause skin and eye irritation. The critical micelle concentration (CMC) in pure water at 25°C is 0.0082 M,[9] and the aggregation number at this concentration is usually considered to be about 62.[10] The micelle ionization fraction (α) is around 0.3 (or 30%).[11]
Evidence suggests that surfactants such as sodium lauryl sulfate can act as a shark repellent at concentrations on the order of 100 parts per million. However, this does not meet the desired "cloud" deterrence level of 0.1 parts per million.[12] [13]
Production

SDS is synthesized by treating lauryl alcohol with sulfur trioxide gas, or oleum, or chlorosulfonic acid to produce hydrogen lauryl sulfate. The industrially practiced method typically uses sulfur trioxide gas. The resulting product is then neutralized through the addition of sodium hydroxide or sodium carbonate.[14] Lauryl alcohol is in turn usually derived from either coconut or palm kernel oil by hydrolysis, which liberates their fatty acids, followed by reduction of the acid group to an alcohol.
Due to this synthesis method, commercially available SDS is actually not pure dodecyl sulfate but a mixture of alkyl sulfates with dodecyl sulfate as the main component.[15]
Toxicology
Carcinogenicity
SDS is not carcinogenic when either applied directly to skin or consumed.[1] A review of the scientific literature stated "SLS [SDS] was negative in an Ames (bacterial mutation) test, a gene mutation and sister chromatid exchange test in mammalian cells, as well as in an in vivo micronucleus assay in mice. The negative results from in vitro and in vivo studies indicate SDS does not interact with DNA."[16]
Sensitivity
It has however been shown to irritate the skin of the face with prolonged and constant exposure (more than an hour) in young adults.[2] SDS may worsen skin problems in individuals with chronic skin hypersensitivity, with some people being affected more than others.[17][18][19] SDS has also been shown to irritate the skin of the face with prolonged and constant exposure (more than an hour) in young adults.[2] In animal studies SDS appears to cause skin and eye irritation.[16]
Aphthous ulcers
A preliminary study suggested SDS in toothpaste caused the recurrence of aphthous ulcers, commonly referred to in some countries as canker sores or white sores.[20] The preliminary study "showed a statistically significant decrease in the number of aphthous ulcers from 14.3 after using the SLS-containing dentifrice to 5.1 ulcers after brushing with the SLS-free dentifrice."[20] A clinical study comparing the incidence of recurrent aphthous ulcers during the use of dentifrices with and without sodium lauryl sulfate supported the findings of an earlier independent study which suggest that use of an SLS-free dentifrice should be considered for individuals who suffer from recurrent aphthous ulcers.[21] A clinical double-blind crossover study found sodium lauryl sulfate had a significantly higher frequency of aphthous ulcers than both cocoamidopropyl betaine or a detergent-free paste, on 30 patients with frequent occurrences of recurrent aphthous ulcers.[3] The clinical double-blind crossover study suggests use of an SLS-free toothpaste for patients with recurrent aphthous ulcers would reduce recurrence.[3] A double-blind crossover trial comparing toothpastes with and without SLS found that it had no significant effect on ulcer patterns.[4]
Taste alteration
Sodium lauryl sulfate diminishes perception of sweetness,[22] an effect commonly observed after recent use of toothpaste containing this ingredient.[23]
See also
References
- ^ a b CIR publication (1983). "Final Report on the Safety Assessment of Sodium Lauryl Sulfate and Ammonium Lauryl Sulfate". International Journal of Toxicology. 2 (7): 127–181. doi:10.3109/10915818309142005..
- ^ a b c Marrakchi S, Maibach HI (2006). "Sodium lauryl sulfate-induced irritation in the human face: regional and age-related differences". Skin Pharmacol Physiol. 19 (3): 177–80. doi:10.1159/000093112. PMID 16679819.
- ^ a b c Herlofson BB, Barkvoll P (1996). "The effect of two toothpaste detergents on the frequency of recurrent aphthous ulcers". Acta Odontol. Scand. 54 (3): 150–3. doi:10.3109/00016359609003515. PMID 8811135.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Healy CM, Paterson M, Joyston-Bechal S, Williams DM, Thornhill MH (1999 Jan). "The effect of a sodium lauryl sulfate-free dentifrice on patients with recurrent oral ulceration". Oral Dis. 5 (1): 39–43. doi:10.1111/j.1601-0825.1999.tb00062.x. PMID 10218040.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ Piret J, Désormeaux A, Bergeron MG. (2002). "Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses". Curr Drug Targets. 3 (1): 17–30. doi:10.2174/1389450023348037. PMID 11899262.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Piret J, Lamontagne J, Bestman-Smith J, Roy S, Gourde P, Désormeaux A, Omar RF, Juhász J, Bergeron MG. (2000). "In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses". J Clin Microbiol. 38 (1): 110–9. PMC 86033. PMID 10618073.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Watanabe K, Imai S, Mori YH (2005). "Surfactant effects on hydrate formation in an unstirred gas/liquid system: An experimental study using HFC-32 and sodium dodecyl sulfate". Chemical Engineering Science. 60 (17): 4846–4857. doi:10.1016/j.ces.2005.03.043.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ The acronym expands to "sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
- ^ P. Mukerjee and K. J. Mysels, "Critical Micelle Concentration of Aqueous Surfactant Systems", NSRDS-NBS 36, US. Government Printing Office, Washington,.D.C., 197 1.
- ^ N.J. Turro. A. Yekta, J. Am. Chem. Soc., 1978, 100, 5951
- ^ Barney L. Bales, Luis Messina, Arwen Vidal, Miroslav Peric, and Otaciro Rangel Nascimento (1998). "Precision Relative Aggregation Number Determinations of SDS Micelles Using a Spin Probe. A Model of Micelle Surface Hydration". J. Phys. Chem. B. 102 (50): 10347–10358. doi:10.1021/jp983364a.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Joseph A. Sisneros and Donald R. Nelson. "The effectiveness of sodium lauryl sulphate as a shark repellent in a laboratory test situation". Environmental Biology of FishesVolume 60, Numbers 1-3, 117-130, DOI: 10.1023/A:1007612002903. Retrieved 2010-08-27.
- ^ Larry J. Smith Jr. "Surfactants as Chemical Shark Repellents: Past, Present, and Future". Journal of Fish Biology Volume 38, Issue 1, pages 105–113, January 1991. Retrieved 2010-08-27.
- ^ "SODIUM LAURYL SULFATE" (Document). Chemical Land21.
{{cite document}}: Unknown parameter|url=ignored (help) - ^ European Pharmacopoeia: Sodium laurilsulfate
- ^ a b NICNAS - Sodium Lauryl Sulfate
- ^ Agner T (1991). "Susceptibility of atopic dermatitis patients to irritant dermatitis caused by sodium lauryl sulphate". Acta Derm. Venereol. 71 (4): 296–300. PMID 1681644.
- ^ Nassif A, Chan SC, Storrs FJ, Hanifin JM (1994). "Abnormal skin irritancy in atopic dermatitis and in atopy without dermatitis". Arch Dermatol. 130 (11): 1402–7. doi:10.1001/archderm.130.11.1402. PMID 7979441.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Löffler H, Effendy I (1999). "Skin susceptibility of atopic individuals". Contact Derm. 40 (5): 239–42. doi:10.1111/j.1600-0536.1999.tb06056.x. PMID 10344477.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Herlofson BB, Barkvoll P (1994). "Sodium lauryl sulfate and recurrent aphthous ulcers. A preliminary study". Acta Odontol. Scand. 52 (5): 257–9. doi:10.3109/00016359409029036. PMID 7825393.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chahine L, Sempson N, Wagoner C (1997). "The effect of sodium lauryl sulfate on recurrent aphthous ulcers: a clinical study". Compend Contin Educ Dent. 18 (12): 1238–40. PMID 9656847.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Adams, Michael J. Characterization and Measurement of Flavor Compounds: Substances That Modify the Perception of Sweetness. ACS Publications, 1985, p.11-25. Abstract online at http://pubs.acs.org/doi/abs/10.1021/bk-1985-0289.ch002
- ^ Clark, Josh. "Why does orange juice taste bad after you brush your teeth?" Discovery Health. http://health.howstuffworks.com/wellness/beauty-hygiene/orange-juice-toothpaste.htm
