Uterine fibroid: Difference between revisions
m Reverted good faith edits by July6177 (talk); Rv, insuficient context to integerate. |
|||
| Line 104: | Line 104: | ||
[[IUD with progestogen|Levonorgestrel intrauterine devices]] are highly effective in limiting menstrual blood flow. Side effects are typically very moderate because the [[levonorgestrel]] (a [[progestin]]) is released in low concentration locally. While most Levongestrel-IUD studies concentrated on treatment of women without fibroids a few reported very good results specifically for women with fibroids including a substantial regression of fibroids.<ref>{{Cite pmid|17726816}}</ref> One reported problem is that women with large fibroids had more frequently spontaneous expulsion of the IUD, however many of those asked for reinsertion of the device indicating a high rate of satisfaction despite the expulsion.<ref name=Sankaran_2008 /><ref>{{Cite pmid|19920976}}</ref> |
[[IUD with progestogen|Levonorgestrel intrauterine devices]] are highly effective in limiting menstrual blood flow. Side effects are typically very moderate because the [[levonorgestrel]] (a [[progestin]]) is released in low concentration locally. While most Levongestrel-IUD studies concentrated on treatment of women without fibroids a few reported very good results specifically for women with fibroids including a substantial regression of fibroids.<ref>{{Cite pmid|17726816}}</ref> One reported problem is that women with large fibroids had more frequently spontaneous expulsion of the IUD, however many of those asked for reinsertion of the device indicating a high rate of satisfaction despite the expulsion.<ref name=Sankaran_2008 /><ref>{{Cite pmid|19920976}}</ref> |
||
[http://www.nobleherb.com/goods-19.html Hysteromyoma Plaster] is a Chinese herbal medicine. It is a kind of external medicine which feature is to treat the internal illness by external treatment. So it avoids the damage to the body by oral drugs and it is much safer. Taking advantage of the good osmosis and efficacy of traditional black plaster, Hysteromyoma Plaster can cure uterine fibroids by apply it to lower abdomen, navel and back of the waist. The principle is through applying the plaster to skin, the medicines permeate the lesion site, have effects on pathogenic factors and cure the disease. |
|||
[[Danazol]] is an effective treatment to shrink fibroids and control symptoms. Its use is limited by unpleasant side effects. Mechanism of action is thought to be antiestrogenic effects. Recent experience indicates that safety and side effect profile can be improved by more cautious dosing.<ref name=Sankaran_2008 /> |
[[Danazol]] is an effective treatment to shrink fibroids and control symptoms. Its use is limited by unpleasant side effects. Mechanism of action is thought to be antiestrogenic effects. Recent experience indicates that safety and side effect profile can be improved by more cautious dosing.<ref name=Sankaran_2008 /> |
||
Revision as of 03:18, 28 December 2010
| Uterine fibroid | |
|---|---|
| Specialty | Oncology |
A uterine fibroid (also uterine leiomyoma,[1] myoma, fibromyoma, leiofibromyoma, fibroleiomyoma, and fibroma) (the plural of myoma is myomas or myomata) is a benign (non-cancerous) tumor that originates from the smooth muscle layer (myometrium) and the accompanying connective tissue of the uterus.
Fibroids are the most common benign tumors in females and typically found during the middle and later reproductive years. While most fibroids are asymptomatic, they can grow and cause heavy and painful menstruation, painful sexual intercourse, and urinary frequency and urgency. Some fibroids may interfere with pregnancy although this appears to be very rare.[2]
In the US, symptoms caused by uterine fibroids are a very frequent indication for hysterectomy.[3] Fibroids are often multiple and if the uterus contains too many leiomyomatas to count, it is referred to as diffuse uterine leiomyomatosis. The malignant version of a fibroid is extremely uncommon and termed a leiomyosarcoma.
Prevalence

About 20–40% of women will be diagnosed with leiomyoma but only a fraction of those will cause problems or require treatment.[3]
The condition is about twice as common in black women as white women.[4][5].
Leiomyoma are more common in overweight women (perhaps because of increased estrogen from adipose aromatase activity).[6] Fibroids are dependent on estrogen and progesterone to grow and therefore relevant only during the reproductive years, they are expected to shrink after menopause.
Pathology and histology
Leiomyomas grossly appear as round, well circumscribed (but not encapsulated), solid nodules that are white or tan, and show whorled appearance on histological section. The size varies, from microscopic to lesions of considerable size. Typically lesions the size of a grapefruit or bigger are felt by the patient herself through the abdominal wall.

Microscopically, tumor cells resemble normal cells (elongated, spindle-shaped, with a cigar-shaped nucleus) and form bundles with different directions (whorled). These cells are uniform in size and shape, with scarce mitoses. There are three benign variants: bizarre (atypical); cellular; and mitotically active.
Location


Growth and location are the main factors that determine if a fibroid leads to symptoms and problems.[3] A small lesion can be symptomatic if located within the uterine cavity while a large lesion on the outside of the uterus may go unnoticed. Different locations are classified as follows:
- Intramural Fibroids are located within the wall of the uterus and are the most common type; unless large, they may be asymptomatic. Intramural fibroids begin as small nodules in the muscular wall of the uterus. With time, intramural fibroids may expand inwards, causing distortion and elongation of the uterine cavity.
- Subserosal fibroids are located underneath the mucosal (peritoneal) surface of the uterus and can become very large. They can also grow out in a papillary manner to become pedunculated fibroids. These pedunculated growths can actually detach from the uterus to become a parasitic leiomyoma.
- Submucosal fibroids are located in the muscle beneath the endometrium of the uterus and distort the uterine cavity; even small lesion in this location may lead to bleeding and infertility. A pedunculated lesion within the cavity is termed an intracavitary fibroid and can be passed through the cervix.
- Cervical fibroids are located in the wall of the cervix (neck of the uterus). Rarely fibroids are found in the supporting structures (round ligament, broad ligament, or uterosacral ligament) of the uterus that also contain smooth muscle tissue.
Fibroids may be single or multiple. Most fibroids start in an intramural location, that is the layer of the muscle of the uterus. With further growth, some lesions may develop towards the outside of the uterus or towards the internal cavity. Secondary changes that may develop within fibroids are hemorrhage, necrosis, calcification, and cystic changes.
Aetiology and pathogenesis
Fibroids are monoclonal tumors, approximately 40 to 50% show karyotypically detectable chromosomal abnormalities. When multiple fibroids are present they will usually have mostly unrelated genetic defects. Exact aetiology is not nearly understood, current working hypothesis is that genetic predispositions, prenatal hormone exposure and the effects of hormones, growth factors and xenoestrogens cause fibroid growth. Known risk factors are African-American descent, nulliparity, obesity, polycystic ovary syndrome, diabetes and hypertension.[7]
Fibroid growth is strongly dependent on estrogen and progesterone. Although both estrogen and progesterone are usually regarded as growth promoting they will also cause growth restriction in some circumstances. Paradoxically fibroids will rarely grow during pregnancy despite very high steroid hormone levels and pregnancy appears to exert a certain protective effect. [2]This protective effect might be partially mediated by an interaction estrogen and the oxytocin receptor.[8]
It is believed that estrogen and progesterone have both mitogenic effect on leiomyoma cells and also act by influencing (directly and indirectly) a large number of growth factors, cytokines and apoptotic factors as well as other hormones. Furthermore the actions of estrogen and progesterone are modulated by the cross-talk between estrogen, progesterone and prolactin signalling which controls the expression of the respective nuclear receptors. It is believed that estrogen is growth promoting by up-regulating IGF-1, EGFR, TGF-beta1, TGF-beta3 and PDGF, promotes aberrant survival of leiomyoma cells by down-regulating p53, increasing expression of the anti-apoptotic factor PCP4 and antagonizing PPAR-gamma signalling. Progesterone is thought to promote the growth of leiomyoma through up-regulating EGF, TGF-beta1 and TGF-beta3, and the survival through up-regulating Bcl-2 expression and down-regulating TNF-alpha. Progesterone is believed to counteract growth by downregulating IGF-1.[9][10][11] Expression of transforming growth interacting factor (TGIF) is increased in leiomyoma compared with myometrium.[12] TGIF is a potential repressor of TGF-β pathways in myometrial cells.[12]
Whereas in premenopausal fibroids the ER-beta, ER-alpha and progesterone receptors are found overexpressed, in the rare postmenopausal fibroids only ER-beta was found significantly overexpressed.[13] Most studies found that polymorphisms in ER and PR gene encodings are not correlated with incidence of fibroids in Caucasian populations [14][15] however a special ER-alpha genotype was found correlated with incidence and size of fibroids. The higher prevalence of this genotype in black women may also explain the high incidence of fibroids in this group.[16]
Uterine leiomyoma was more sensitive than normal myometrium to PPAR-gamma receptor activation resulting in reduced survival and apoptosis of leiomyoma cells. The mechanism is thought to involve negative cross-talk between ER and PPAR signaling pathways. Several PPAR-gamma ligands were considered as potential treatment.[17] PPAR-gamma agonists may also counteract leiomyoma growth by several other mechanisms of action such as TGF-beta3 expression inhibition.[18]
Hypertension is significantly correlated with fibroids. Although a causal relationships is not at all clear the hypothesis has been formulated that atherosclerotic injury to uterine blood vessels and the resulting inflammatory state may play a role. Furthermore endocrine factors related to blood pressure such as angiotensin II are suspected to cause fibroid proliferation via angiotensin II type 1 receptor. [19][20]
Aromatase and 17beta-hydroxysteroid dehydrogenase are aberrantly expressed in fibroids, indicating that fibroids can convert circulating androstenedione into estradiol. [21] Similar mechanism of action has been elucidated in endometriosis and other endometrial diseases. [22] Aromatase inhibotors are currently considered for treatment, at certain doses they would completely inhibit estrogen production in the fibroid while not largely affecting ovarian production of estrogen (and thus systemic levels of it). Aromatase overexpression is particularly pronounced in Afro-American women [23]
Genetic and hereditary causes are being considered and several epidemiologic findings indicate considerable genetic influence especially for early onset cases. First degree relatives have a 2.5-fold risk, and nearly 6-fold risk when considering early onset cases. Monozygotic twins have double concordance rate for hysterectomy compared to dizygotic twins.[24]
Like keloids, fibroids have disregulated production of extracellular matrix. Recent studies suggest that this production may represent an abnormal response to ischemic and mechanical tissue stress.[25] Several factors indicate significant involvement of extracellular signaling pathways such as ERK1 and ERK2, which in fibroids are prominently influenced by hormones.[26] Paradoxically and unlike most other conditions involving significant fibrosis the Cyr61 gene has been found downregulated in fibroids.[27]
Cyr61 is also known for its role as tumor suppressing factor and in angiogenesis. Hence fibroids are one of the very few tumors with reduced vascular density. [27]
Symptoms
Generally, symptoms relate to the location of the lesion and its size (mass effect). Important symptoms include abnormal gynecologic hemorrhage, heavy or painful periods, abdominal discomfort or bloating, painful defecation, back ache, urinary frequency or retention, and in some cases, infertility.[28] There may also be pain during intercourse, depending on the location of the fibroid. During pregnancy they may be the cause of miscarriage, bleeding, premature labor, or interference with the position of the fetus.
Fibroids, particularly when small, may be entirely asymptomatic. The U.S. Department of Health & Human Services states that "Fibroids are almost always benign (not cancerous). Rarely (less than one in 1,000) a cancerous fibroid will occur. This is called leiomyosarcoma. Doctors think that these cancers do not arise from an already-existing fibroid. Having fibroids does not increase the risk of developing a cancerous fibroid. Having fibroids also does not increase a woman's chances of getting other forms of cancer in the uterus."[29]
While fibroids are common, they are not a typical cause for infertility accounting for about 3% of reasons why a woman may not have a child.[30] Typically in such cases a fibroid is located in a submucosal position and it is thought that this location may interfere with the function of the lining and the ability of the embryo to implant.[30] Also larger fibroids may distort or block the fallopian tubes.
Diagnosis
While a bimanual examination typically can identify the presence of larger fibroids, gynecologic ultrasonography (ultrasound) has evolved as the standard tool to evaluate the uterus for fibroids. Sonography will depict the fibroids as focal masses with a heterogeneous texture, which usually cause shadowing of the ultrasound beam. The location can be determined and dimensions of the lesion measured. Also magnetic resonance imaging (MRI) can be used to define the depiction of the size and location of the fibroids within the uterus.
Imaging modalities cannot clearly distinguish between the benign uterine leiomyoma and the malignant uterine leiomyosarcoma, however, the latter is quite rare. However fast growth or unexpected growth such as enlargement of a lesion after the menopause raise the level of suspicion that the lesion might be a sarcoma. Also, with advanced malignant lesions there may be evidence of local invasion. A more recent study has suggested that diagnostic capabilities using MRI have improved the ability to detect sarcomatous lesions.[31] Biopsy is rarely performed and if performed, is rarely diagnostic. Should there be an uncertain diagnosis after ultrasounds and MRI imaging, surgery is generally indicated.
Other imaging techniques that may be helpful specifically in the evaluation of lesions that affect the uterine cavity are hysterosalpingography or sonohysterography.
Coexisting disorders
Fibroids that lead to heavy vaginal bleeding lead to anemia and iron deficiency. Due to pressure effects gastrointestinal problems are possible such as constipation and bloatedness. Compression of the ureter may lead to hydronephrosis. Fibroids may also present alongside endometriosis, which itself may cause infertility. Adenomyosis may be mistaken for or coexist with fibroids.
In very rare cases, malignant (cancerous) growths, leiomyosarcoma, of the myometrium can develop.[32]
Treatment
Most fibroids do not require treatment unless they are causing symptoms. After menopause fibroids shrink and it is unusual for fibroids to cause problems.
Symptomatic uterine fibroids can be treated by:
- medication to control symptoms
- medication aimed at shrinking tumours
- ultrasound fibroid destruction
- various surgically aided methods to reduce blood supply of fibroids
- myomectomy or radio frequency ablation
- hysterectomy
Herbal treatment
Most frequently used herbal treatments are Vitex agnus-castus, Yarrow and Capsella bursa-pastoris. There is no clinical evidence supporting their use in the treatment of fibroids, however for Vitex[33] and Yarrow there is relatively solid evidence that they can reduce menstrual bleeding and PMS symptoms.
Medication
A number of medications are in use to control symptoms caused by fibroids. NSAIDs can be used to reduce painful menses. Oral contraceptive pills are prescribed to reduce uterine bleeding and cramps. [30] Anemia may have to be treated with iron supplementation.
Levonorgestrel intrauterine devices are highly effective in limiting menstrual blood flow. Side effects are typically very moderate because the levonorgestrel (a progestin) is released in low concentration locally. While most Levongestrel-IUD studies concentrated on treatment of women without fibroids a few reported very good results specifically for women with fibroids including a substantial regression of fibroids.[34] One reported problem is that women with large fibroids had more frequently spontaneous expulsion of the IUD, however many of those asked for reinsertion of the device indicating a high rate of satisfaction despite the expulsion.[35][36]
Hysteromyoma Plaster is a Chinese herbal medicine. It is a kind of external medicine which feature is to treat the internal illness by external treatment. So it avoids the damage to the body by oral drugs and it is much safer. Taking advantage of the good osmosis and efficacy of traditional black plaster, Hysteromyoma Plaster can cure uterine fibroids by apply it to lower abdomen, navel and back of the waist. The principle is through applying the plaster to skin, the medicines permeate the lesion site, have effects on pathogenic factors and cure the disease.
Danazol is an effective treatment to shrink fibroids and control symptoms. Its use is limited by unpleasant side effects. Mechanism of action is thought to be antiestrogenic effects. Recent experience indicates that safety and side effect profile can be improved by more cautious dosing.[35]
Dostinex in a moderate and well tolerated dosis has been shown in 2 studies to shrink fibroids effectively. Mechanism of action is completely unclear.[35][37]
Gonadotropin-releasing hormone analogs cause temporary regression of fibroids by decreasing estrogen levels. Because of the limitations and side effects of this medication it is rarely recommended other than for preoperative use to shrink the size of the fibroids and uterus before surgery. Its is typically used for a maximum of 6 months or shorter because after longer use they could cause osteoporosis and other typically postmenopausal complications. The main side effects are transient postmenopausal symptoms. In many cases the fibroids will regrow after cessation of treatment, however significant benefits may persists for much longer time in some cases. Several variations are possible, such as GnRH agonists with add-back regimens intended to decrease the adverse effects of estrogen deficiency. Several add-back regimes are possible, tibolone, raloxifene, progestogens alone, estrogen alone, and combined estrogens and progestogens.[35]
Aromatase inhibitors have been used experimentally to reduce fibroids. The effect is believed to be due partially by lowering systemic estrogen levels and partially by inhibiting locally overexpressed aromatase in fibroids.[35] Experience from experimental aromatase inhibitor treatment of endometriosis indicates that aromatase inhibitors might be particularly useful in combination with a progestogenic ovulation inhibitor.
Progesterone antagonists have been shown in small studies to decrease the size of uterine fibroids. Mifepristone was effective in a placebo-controlled pilot study.[38] [39]Selective progesterone receptor modulators, such as Progenta, have been under investigation.
The selective progesterone receptor modulator Asoprisnil is currently tested with very promising results as a possible use as a treatment for fibroids - the hope is that it will provide the advantages of progesterone antangonitst without their adverse effects.[35]
The long term safety of progesterone antagonists as well as selective progesterone receptor modulators has yet to be established.[40][41]
Magnetic Resonance-Guided Focused Ultrasound
Magnetic Resonance guided Focused Ultrasound (MRgFUS), is a non-invasive intervention (requiring no incision) that uses high intensity focused ultrasound (HIFU) waves to ablate (destroy) tissue in combination with Magnetic Resonance Imaging (MRI), which guides and monitors the treatment. This technique was approved by the FDA in 2004. Ultrasound is a form of energy that passes through skin, muscle, fat and other soft tissue. High intensity focused ultrasound energy, focused on a small target volume (tumor), provides a therapeutic effect by raising the tissue temperature of the target (tumor) high enough to destroy it. This is similar to the manner in which sunlight focused by a magnifying glass can create sufficient heat to start a fire. The use of heat to destroy tissue is called thermal ablation. Treatments consist of multiple exoposures of focused energy or sonications. MRgFUS uses a Magnetic Resonance Imaging (MRI) scanner to identify tissues in the body and plan the treatment. During the procedure, delivery of focused ultrasound energy is guided and controlled using MR thermal imaging. • MR imaging provides a three-dimensional view of the target tissue, allowing for precise focusing of ultrasound energy within a desired volume. • Additionally, the MR imaging provides quantitative, real-time, thermal images of the treated area. This allows the physician to ensure that the temperature generated during each cycle of ultrasound energy is sufficient to cause thermal ablation within the desired tissue and if not, to adapt the parameters. The advantage and value of MR guidance ensures safe and accurate treatment. [42] • Patients who have symptomatic fibroids, who desire a non-invasive treatment option and who do not have contraindictions for MRI are candidates for MRgFUS. About 60% of patients qualify. It is an outpatient procedure and takes one to three hours depending on the size of the fibroids. It is safe and effective [43]. For patients who have a total fibroid volume of more than 500cc, they may be pretreated for 3 months with a gonadotropin-releasing hormone (GnRH) analogue (e.g. leuprorelin) to reduce leiomyoma size and subsequently, improve thermal ablation efficacy[44]. Fibroid characteristics (T2 hypointensity, smaller size, intramural location, fewer number) are important predictor of treatment success [45][46][47][48]. Symptomatic improvement is sustained for two plus years. [49]. Need for additional treatment varies from 16-20% and is largely dependent on the amount of fibroid that can be safely ablated; the higher the ablated volume, the lower the re-treatment rate[50]. • In comparison to available treatment options, the cost effectiveness of MRgFUS in the U.S. and U.K. has been found to be reasonable and comparable to alternative treatments (hysterectomy, pharmacotherapy, uterine artery embolization)[51][52]. • The largest hurdle for patients who desire MRgFUS is insurance coverage. Most insurers will not cover MRgFUS even though there is a large body of evidence to support its efficacy and safety. The most likely reason is because there are no randomized trial between MRgFUS and UAE. A multi-center trial is underway to investigate the efficacy of MRgFUS vs. UAE.
Uterine artery embolization
Uterine artery embolization (UAE): Using interventional radiology techniques, the interventional radiologist occludes both uterine arteries, thus reducing blood supply to the fibroid[53] . A small catheter (1 mm in diameter) is inserted into the femoral artery at the level of the groin under local anesthesia. Under imaging guidance, the interventional radiologist will enter selectively into both uterine arteries and inject small (500 µm) particles that will block the blood supply to the fibroids. A patient will usually recover from the procedure within a few days. The UAE procedure should result in limited blood supply to the fibroids which should prevent them from further growth, heavy bleeding and possibly shrink them.
A retrospective cohort study showed that UAE has much fewer serious adverse effects than hysterectomy (odds ratio 0.25) and similar rates of satisfaction. In this study, 86% of women treated with UAE would recommend the treatment to a friend compared to 70% of those treated by hysterectomy.[54]
Uterine artery ligation
Uterine artery ligation, sometimes also laparoscopic occlusion of uterine arteries are minimaly invasive methods to limit blood supply of the uterus by a small surgery that can be performed transvaginally or laparoscopically. The principal mechanism of action may be similar like in UAE. This is a relatively new method which demonstrated similar efficacy similar like UAE but is easier to perform and for this reason fewer side effects are expected.[55][56][57] UAE currently appears much more effective than this method in direct comparison.Cite error: The <ref> tag has too many names (see the help page).
Radio frequency ablation
Radiofrequency ablation: One of the newest minimally invasive treatments for fibroids is radiofrequency ablation [58]. In this technique the fibroid is shrunk by inserting a needle-like device into the fibroid through the abdomen and heating it with radio-frequency (RF) electrical energy to cause necrosis of cells. The treatment is a potential option for women who have fibroids, have completed child-bearing and want to avoid a hysterectomy.
Surgery

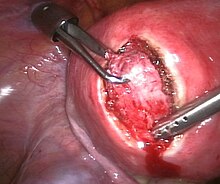
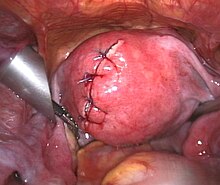
It is possible to remove multiple fibroids during a myomectomy. Although a myomectomy cannot prevent the recurrence of fibroids at a later date, such surgery is increasingly recommended, especially in the case of women who have not completed bearing children or who express an explicit desire to retain the uterus. There are three different types of myomectomy:
- In a hysteroscopic myomectomy, the fibroid is removed by the use of a resectoscope, an endoscopic instrument that can use high-frequency electrical energy to cut tissue. Hysteroscopic myomectomies can be done as an outpatient procedure, with either local or general anesthesia used. Hysteroscopic myomectomy is most often recommended for submucosal fibroids. A French study collected results from 235 patients suffering from submucous myomas who were treated with hysteroscopic myomectomies; in none of these cases was the fibroid greater than 5 cm.[59]
- A laparoscopic myomectomy requires a small incision near the navel. The physician then inserts a laparoscope into the uterus and uses surgical instruments to remove the fibroids. Studies have suggested that laparoscopic myomectomy leads to lower morbidity rates and faster recovery than does laparotomic myomectomy.[60] As with hysteroscopic myomectomy, laparoscopic myomectomy is not generally used on very large fibroids. A study of laparoscopic myomectomies conducted between January 1990 and October 1998 examined 106 cases of laparoscopic myomectomy, in which the fibroids were intramural or subserous and ranged in size from 3 to 10 cm.[61]
- A laparotomic myomectomy (also known as an open or abdominal myomectomy) is the most invasive surgical procedure to remove fibroids. The physician makes an incision in the abdominal wall and removes the fibroid from the uterus. A particularly extensive laparotomic procedure may necessitate that any future births be conducted by Caesarean section.[30] Recovery time from a laparatomic procedure is generally expected to be four to six weeks.
Hysterectomy is the classical method of treating fibroids. Although it is now recommended only as last option it is still the leading cause of hysterectomies in the US.
Endometrial ablation
Endometrial ablation can be used if the fibroids are only within the uterus and not intramural and relatively small. High failure and recurrence rates are expected in the presence of larger or intramural fibroids.
Other
The use of vitex herbal medicine lacks supporting evidence.
Malignancy
About 1 out of 1000 lesions[30] are or become malignant, typically as a leiomyosarcoma on histology. A sign that a lesion may be malignant is growth after menopause.[30] There is no consensus among pathologists regarding the transformation of Leiomyoma into a sarcoma. Most pathologists believe that a Leiomyosarcoma is a de novo disease[citation needed].
Metastasis
There are a number of rare conditions in which fibroids metastasize. They still grow in a benign fashion, but can be dangerous depending on their location.[62]
- In leiomyoma with vascular invasion, an ordinary-appearing fibroid invades into a vessel but there is no risk of recurrence.
- In Intravenous leiomyomatosis, leiomyomata grow in veins with uterine fibroids as their source. Cardiac involvement can be fatal.
- In benign metastasizing leiomyoma, leiomyomata grow in more distant sites such as the lungs and lymph nodes. The source is not entirely clear. Pulmonary involvement can be fatal.
- In disseminated intraperitoneal leiomyomatosis, leiomyomata grow diffusely on the peritoneal and omental surfaces, with uterine fibroids as their source. This can simulate a malignant tumor but behaves benignly.
Organizations, research and political aspects
The Center for Uterine Fibroids which is associated with Brigham and Women’s Hospital in Boston MA ([1]) is currently recruiting volunteers for an investigation of all aspects of the aetiology, pathology of fibroids and development of treatment options. This institution also has a lot of clinical trials focused on African American women. The focus of one study is the search for a specific gene associated with the development of fibroids. Since one risk factor for uterine fibroids is having a family history of fibroids, the results of this study will provide some answers on the heredity of the illness. Some women may interact with the healthcare system by getting an ultrasound to diagnosis symptoms if they’ve had a mother, sister or grandmother who has previously suffered with these benign tumors.
Fibroid Research and Education Act of 2005
The 2005 S.1289 bill was read twice and referred to the committee on Health, Labor and Pensions but never passed for a Senate or House vote. The proposed Uterine Fibroid Research and Education Act of 2005 mentioned that $5 billion dollars is spent annually on hysterectomy surgeries each year, which affect 22% of African Americans and 7% of Caucasian women. The bill also called for more funding for research and educational purposes. It also states that of the $27 million dollars issued to NIH, only $5 million was allocated for Uterine Fibroids in 2004.
Fibroids in animals
Uterine fibroids are rare in animals, although they have been observed in certain dogs and Baltic Gray Seals.[63]
See also
References
- ^ "uterine leiomyoma" at Dorland's Medical Dictionary
- ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17039693, please use {{cite journal}} with
|pmid=17039693instead. - ^ a b c Wallach EE, Vlahos NF. "Uterine myomas: an overview of development, clinical features, and management". Obstet Gynecol 104 (2004), pp. 393–406.
- ^ Uterine Fibroids – The Merck Manuals Online Medical Library
- ^ Wise, L., Palmer, J., Bernard, H., Stewart, E., Rosenberg, L., (2005) Age-Specific Incidence rates for Self-Reported Uterine Leiomyomata in the Black Women’s Health Study Obstet Gynecol 105(3): 563-568
- ^ Uterine Fibroids – The Merck Manuals Online Medical Library
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18534913, please use {{cite journal}} with
|pmid=18534913instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12517588 , please use {{cite journal}} with
|pmid=12517588instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11035984, please use {{cite journal}} with
|pmid=11035984instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15140868, please use {{cite journal}} with
|pmid=15140868instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17407572, please use {{cite journal}} with
|pmid=17407572instead. - ^ a b Yen-Ping Ho J, Man WC, Wen Y, Polan ML, Shih-Chu Ho E, Chen B (2009). "Transforming growth interacting factor expression in leiomyoma compared with myometrium". Fertil. Steril. 94 (3): 1078–83. doi:10.1016/j.fertnstert.2009.05.001. PMC 2888713. PMID 19524896.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17646097, please use {{cite journal}} with
|pmid=17646097instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12552233, please use {{cite journal}} with
|pmid=12552233instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19639498, please use {{cite journal}} with
|pmid=19639498instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16860797, please use {{cite journal}} with
|pmid=16860797instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17893092, please use {{cite journal}} with
|pmid= 17893092instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18272045, please use {{cite journal}} with
|pmid= 18272045instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15781952, please use {{cite journal}} with
|pmid=15781952instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17993476, please use {{cite journal}} with
|pmid=17993476instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15083381, please use {{cite journal}} with
|pmid=15083381instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11850203, please use {{cite journal}} with
|pmid=11850203instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19240151, please use {{cite journal}} with
|pmid= 19240151instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17613550, please use {{cite journal}} with
|pmid=17613550instead. - ^ Payson M, Malik M, Siti-Nur Morris S, Segars JH, Chason R, Catherino WH. (2009). "Activating transcription factor 3 gene expression suggests that tissue stress plays a role in leiomyoma development". Fertil Steril. 2009 Aug;92(2):748-55. Epub 2008 Aug 9. 92 (2): 748–55. doi:10.1016/j.fertnstert.2008.06.030. PMID 18692824.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19179429, please use {{cite journal}} with
|pmid=19179429instead. - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12900513, please use {{cite journal}} with
|pmid= 12900513instead. - ^ Fibroid Tumors
- ^ http://www.4woman.gov/faq/uterine-fibroids.cfm#1
- ^ a b c d e f American Society of Reproductive Medicine Patient Booklet: Uterine Fibroids, 2003
- ^ Goto A, Takeuchi S, Sugimura K, Maruo T (2002). "Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus". Int. J. Gynecol. Cancer. 12 (4): 354–61. doi:10.1046/j.1525-1438.2002.01086.x. PMID 12144683.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fibroids – What is it? – Introduction
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15783241, please use {{cite journal}} with
|pmid=15783241instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17726816, please use {{cite journal}} with
|pmid=17726816instead. - ^ a b c d e f Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.bpobgyn.2008.03.001, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.bpobgyn.2008.03.001instead. http://www.britishfibroidtrust.org.uk/journals/bft_Sankaran.pdf - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19920976, please use {{cite journal}} with
|pmid=19920976instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19668882, please use {{cite journal}} with
|pmid=19668882instead. - ^ Engman M, Granberg S, Williams ARW, Meng CX, Lalitkumar PGL, Gemzell-Danielsson K (August 2009). "Mifepristone for Treatment of Uterine Leiomyoma. A Prospective Randomized Placebo Controlled Trial". Human Reproduction 24 (8): 1870–9. 24 (8): 1870–9. doi:10.1093/humrep/dep100. PMID 19389793.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18539512, please use {{cite journal}} with
|pmid=18539512instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19176543, please use {{cite journal}} with
|pmid=19176543instead. - ^ http://www.apmhealtheurope.com/story.php?include_profilDepsPage=197&numero=283&profil=94&ctx=6a15602f32780ba0e6037b5e94f2b272
- ^ "FDA Approves New Device to Treat Uterine Fibroids" (Press release). FDA. 2004-10-22. Retrieved 2008-05-26.
- ^ [Stewart, E.A., et al., Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol, 2003. 189(1): p. 48-54.]
- ^ [Smart, O.C., et al., Gonadotrophin-releasing hormone and magnetic-resonance-guided ultrasound surgery for uterine leiomyomata. Obstet Gynecol, 2006. 108(1): p. 49-54.]
- ^ [LeBlang, S.D., Patient Selection for MRgFUS in the Treatment of Uterine Fibroids, in MR-guided Focused Ultrasound 2010 2nd International Symposium. 2010: Washington D.C. p. 116.]
- ^ [Yoon, S.W., et al., Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol, 2008. 18(12): p. 2997-3006.]
- ^ []
- ^ [FDA. Summary of Safety and Effectiveness Data. June 3, 2004 [cited 2004; Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf4/P040003b.pdf.]
- ^ [Stewart, E.A., et al., Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril, 2006. 85(1): p. 22-9.]
- ^ [Kurashvili, J., et al. MRgFUS Treatment for Uterine Myomas: Safety, Effectiveness and Pathogenesis. in MR-guided Focused Ultrasound 2010 2nd International Symposium. 2010. Washington, D.C.]
- ^ [O'Sullivan, A.K., et al., Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids. Int J Technol Assess Health Care, 2009. 25(1): p. 14-25.]
- ^ [Zowall, H., et al., Cost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroids. BJOG, 2008. 115(5): p. 653-62.]
- ^ The Embolisation Process: What's Involved
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18331704, please use {{cite journal}} with
|pmid=18331704instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11172850, please use {{cite journal}} with
|pmid=11172850instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19178748, please use {{cite journal}} with
|pmid=19178748instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16168993, please use {{cite journal}} with
|pmid=16168993instead. - ^ Beck, Melinda (2010-01-20). "A New Treatment to Help Women Avoid Hysterectomy". The Wall Street Journal.
- ^ Polena, V., et al. "Long-term results of hysteroscopic myomectomy in 235 patients." European Journal of Obstetrics & Gynecology and Reproductive Biology 130 (2007): 232–237.
- ^ Agdi, M. and Tulandi, T. “Endoscopic management of uterine fibroids.” Best Practice & Research Clinical Obstetrics & Gynecology, online publication 4 Mar 2008.
- ^ Soriano, D. et al. "Pregnancy outcome after laparoscopic and laparoconverted myomectomy." European Journal of Obstetrics & Gynecology and Reproductive Biology 108 (2003): 194–198.
- ^ Fletcher's Diagnostic Histopathology of Tumors, 3rd Ed, p. 692-694.
- ^ Baltic Gray Seals and fibroids
